Abstract
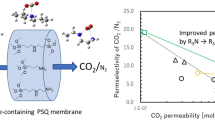
Urea- and isocyanurate-containing polysilsesquioxane (PSQ) membranes were prepared and their CO2 separation performance was investigated. Applying the sol–gel process to N,N’-bis(triethoxysilylpropyl)urea (BTESPU) gave a urea-containing PSQ membrane on an inorganic support, which showed good CO2/N2 permselectivity of 12 with CO2 permeance of 3.8 × 10−9 mol/(m2 s Pa). The copolymerization of BTESPU with bis(triethoxysilyl)alkanes (EtO)3Si(CH2)xSi(OEt)3 (x = 1–3) produced membranes with improved performance, and the membrane prepared with bis(triethoxysilyl)ethane (BTESE, x = 2) showed CO2/N2 permselectivity of 13 and CO2 permeance of 2.2 × 10−7 mol/(m2 s Pa). The homopolymerization of isocyanurate-containing precursors, tris(triethoxysilylpropyl) isocyanurate (TTESPI), tris(triethoxysilylmethyl) isocyanurate (TTESMI), and bis(triethoxysilylpropyl) isocyanurate (BTESPI), was found to yield membranes that have higher performance than the BTESPU homopolymer membrane. Of these, the membrane prepared from TTESPI showed the highest CO2 permeance of 3.2 × 10−7 mol/(m2 s Pa) and the highest CO2/N2 permselectivity of 18. The copolymerization of TTESPI with BTESE resulted in the increase of CO2 permeance by 1.7 times, although CO2/N2 permselectivity was slightly decreased to 12. The CO2-philicity of the urea units, which was estimated by density functional theory calculations on model systems, suggested a relationship between urea structure and membrane performance.
Graphical abstract

Urea- and isocyanurate-containing polysilsesquioxane membranes were prepared and their CO2 separation performance was investigated.
Highlights
-
Urea- and isocyanurate-containing PSQ membranes were prepared.
-
The membranes showed CO2-separation properties.
-
Relationship between membrane structure and separation properties was investigated.










Similar content being viewed by others
References
Cui H, Xie Y, Ye Y, Shi Y, Liang B, Chen B (2021) An ultramicroporous metal-organic framework with record high selectivity for inverse CO2/C2H2 separation. Bull Soc Chem Jpn 94:2689–2701. https://doi.org/10.1246/bcsj.20210237
Wang J, Zhang Y, Su Y, Liu X, Zhang P, Lin RB, Chen S, Deng Q, Zeng Z, Deng S, Chen B (2022) Fine pore engineering in a series of isoreticular metal-organic frameworks for efficient C2H2/CO2 separation. Nat Commun 13:200. https://doi.org/10.1038/s41467-021-27929-7
Robeson LM (2008) The upper bound revisited. J Membr Sci 320:390–400. https://doi.org/10.1016/j.memsci.2008.04.030
Brunetti A, Scura F, Barbieri G, Drioli E (2010) Membrane technologies for CO2 separation. J Membr Sci 359:115–125. https://doi.org/10.1016/j.memsci.2009.11.040
Ma C, Wang M, Wang Z, Gao M, Wang J (2020) Recent progress on thin film composite membranes for CO2 separation. J CO2 Utilization 42:101296. https://doi.org/10.1016/j.jcou.2020.101296
Yu L, Kanezashi M, Nagasawa H, Tsuru T (2018) Role of amine type in CO2 separation performance within amine functionalized silica/organosilica membranes: A review. Appl Sci 8:1032. https://doi.org/10.3390/app8071032
Yu L, Kanezashi M, Nagasawa H, Tsuru T (2017) Fabrication and CO2 permeation properties of amine-silica membranes using a variety of amine types. J Membr Sci 541:447–456. https://doi.org/10.1016/j.memsci.2017.07.024
Xomeriakis G, Tsai C-Y, Brnker CJ (2005) Microporous sol–gel derived aminosilicate membrane for enhanced carbon dioxide separation. Sep Purif Technol 42:249–257. https://doi.org/10.1016/j.seppur.2004.08.003
Paradis GG, Kreiter R, van Tuel MM, Nijmeijer A, Vente JF (2012) Amino-functionalized microporous hybrid silica membranes. J Mater Chem 22:7258–7264. https://pubs.rsc.org/en/content/articlelanding/2012/JM/c2jm15417j
Yu, Kanezashi M, Nagasawa H, Oshita J, Naka A, Tsuru T (2017) Pyrimidine-bridged organoalkoxysilane membrane for high-efficiency CO2 transport via mild affinity. Sep Purif Technol 178:232–241. https://doi.org/10.1016/j.seppur.2017.01.039
Yu L, Kanezashi M, Nagasawa H, Moriyama N, Tsuru T, Ito K (2018) Enhanced CO2 separation performance for tertiary amine-silica membranes via thermally induced local liberation of CH3Cl. AIChE J 64:1528–1539. https://doi.org/10.1002/aic.16040
Guo M, Kanazashi M, Nagasawa H, Yu L, Ohshita J, Tsuru T (2020) Amino-decorated organosilica membranes for highly permeable CO2 capture. J Membr Sci 611:118328. https://doi.org/10.1016/j.memsci.2020.118328
Ren X, Kanezashi M, Guo M, Xu R, Zhong J, Tsuru T (2021) Multiple amine-contained POSS-functionalized organosilica membranes for gas separation. Membranes 11:194, https://www.mdpi.com/2077-0375/11/3/194
Takahashi T, Tanimoto R, Isobe T, Matsushita S, Nakajima A (2016) Surface modification of porous alumina filters for CO2 separation using silane coupling agents. J Membr Sci 497:216–220. https://doi.org/10.1016/j.memsci.2015.09.007
Karimi S, Mortazavi Y, Khodadadi AA, Holmgren A, Korelskiy D, Hedlund J (2020) Functionalization of silica membranes for CO2 separation. Sep Purif Technol 235:116207. https://doi.org/10.1016/j.seppur.2019.116207
Mizumo T, Muragishi H, Yamamoto K, Ohshita J, Kanezashi M, Tsuru T (2015) Preparation and separation properties of oxalylurea-bridged silica membranes. Appl Organomet Chem 29:433–438. https://doi.org/10.1002/aoc.3311
Tsuru T, Nakasuji T, Oka M, Kanezashi M, Yoshioka T (2011) Preparation of hydrophobic nanoporous methylated SiO2 membranes and application to nanofiltration of hexane solutions. J Membr Sci 384:149–156. https://doi.org/10.1016/j.memsci.2011.09.018
Xu R, Wang JH, Kanezashi M, Yoshioka T, Tsuru T (2013) Reverse osmosis performance of organosilica membranes and comparison with the pervaporation and gas permeation properties. AIChE J 59:1298–1307. https://doi.org/10.1002/aic.13885
Kamitani T, Ishida A, Imoto H, Naka K (2021) Supramolecular organogel of polyureas containing POSS units in the main chain: Dependence on the POSS and comonomer structures. Polym J 54:161–167. https://www.nature.com/articles/s41428-021-00578-9
Bergsman DS, Closser RG, Tassone CJ, Clemens BM, Nordlund D, Bent SF (2017) Effect of backbone chemistry on the structure of polyurea films deposited by molecular layer deposition. Chem Mater 29:1192–1203. https://pubs.acs.org/doi/10.1021/acs.chemmater.6b04530
Villaluenga JPG, Seoane B (2001) Experimental estimation of gas-transport properties of linear low-density polyethylene membranes by an integral permeation method. J Appl Polym Sci 82:3013–3021. https://doi.org/10.1002/app.2156
Ariga K (2021) Nanoarchitectonics: What’s coming next after nanotechnology? Nanoscale Horiz 6:364–378. https://pubs.rsc.org/en/content/articlelanding/2021/NH/D0NH00680G
Guo M, Kanezashi M, Nagasawa H, Yu L, Ohshita J, Tsuru T (2020) Amino-decorated organosilica membranes for highly permeable CO2 capture. J Membr Sci 611:118328. https://doi.org/10.1016/j.memsci.2020.118328
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kajimura, K., Horata, K., Adachi, Y. et al. Preparation of urea- and isocyanurate-containing polysilsesquioxane membranes for CO2 separation. J Sol-Gel Sci Technol 106, 149–157 (2023). https://doi.org/10.1007/s10971-022-06004-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-022-06004-7