Abstract
The optical properties of a precursor dry Ferritin powder and its iron oxide derivatives were studied using UV–visible spectroscopy. The iron oxide derivatives were synthesized by applying controlled heat treatment up to 700 °C. The optical properties show dramatic variation in absorption spectra through a large shift in absorption band edge as a function of annealing temperature. On one side (40 ℃) we have precursor Ferritin powder and on the other side (700 ℃) a crystalline hematite phase along with disordered phases at intermediate temperatures. Energy dispersive x-ray (EDX) analysis and XRD reveal that crystallization and thermal destruction of organic cage are inter-related. As ferrihydrite complexes inside the Ferritin cavity go through solidification from amorphous Fe2O3 to hematite crystalline phase (α-Fe2O3), the heterogeneity of electronic excitation processes occurs through ligand-to-metal charge transfer (LMCT), pair excitation and ligand field (d-d) transition. Strong magnetic induction in the intermediate range (400–500 °C) was associated with the enhancement of the pair excitation process. As the material changes phases due to annealing, a large absorption band edge shift from 2.65 to 1.28 eV, a total red shift of 1.37 eV, provides a simple approach to tuneable optical properties of iron oxides.
Graphical abstract

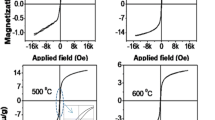
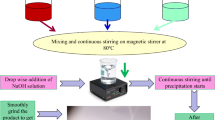
Top panel shows the variation of optical band gap, whereas bottom panel describe the weight percentage variation of different elements with annealing temperature. Middle panel presents the entire theme in form of a sketch of the process related to the synthesis of different phases.
Highlights
-
Ferritin, a biomacromolecule, was used to synthesize nanomaterials with variable optical properties in a sol–gel technique.
-
The variation originates from the structure of nanomaterials which include disordered and crystalline phase of Fe2O3 as a function of annealing temperature.
-
The presence of a large tunable range (1.37 eV) of band gap that covers the entire visible range from blue to red end of the spectrum.
-
EDX and XRD measurements show correlation between thermal destruction of the organic core and crystallization of hematite phase.
-
The pair-excitation transition becomes stronger than LMCT in mid temperature range (400–500 °C) as result of strong magnetic coupling.







Similar content being viewed by others
References
He YP, Miao YM, Li CR et al. (2005) Size and structure effect on optical transitions of iron oxide nanocrystals. Phys Rev B - Condens Matter Mater Phys 71:1–9. https://doi.org/10.1103/PhysRevB.71.125411
MacHala L, Tuček J, Zbořil R (2011) Polymorphous transformations of nanometric iron(III) oxide: a review. Chem Mater 23:3255–3272. https://doi.org/10.1021/cm200397g
Ivantsov R, Ivanova O, Zharkov SM et al. (2020) Magnetic circular dichroism in the canted antiferromagnet α-Fe2O3: bulk single crystal and nanocrystals. J Magn Magn Mater 498:166208. https://doi.org/10.1016/j.jmmm.2019.166208
Bhowmik RN, Sarvanan A (2010) Surface magnetism, Morin transition, and magnetic dynamics in antiferromagnetic α-Fe2O3 (hematite) nanograins. J Appl Phys 107:053916-1–10
Frandsen C, Mørup S (2005) Spin rotation in α-Fe2O3 nanoparticles by interparticle interactions. Phys Rev Lett 94(1–4):027202
Kumar S, Thakur A, Gupta SK et al. (2020) A facile route to synthesis of ferromagnetic and antiferromagnetic phases of iron oxide nanoparticles by controlled heat treatment of ferritin. J Supercond Nov Magn 33:3841–3852. https://doi.org/10.1007/s10948-020-05649-1
Sharma P, Dhiman S, Kumari S, et al. (2019) Revisiting the physiochemical properties of Hematite (-Fe2O3) nanoparticle and exploring its bio-environmental application. Mater Res Express 6. https://doi.org/10.1088/2053-1591/ab30ef
Sivula K, Zboril R, Le Formal F et al. (2010) Photoelectrochemical water splitting with mesoporous hematite prepared by a solution-based colloidal approach. J Am Chem Soc 132:7436–7444. https://doi.org/10.1021/ja101564f
Kumar P, Rawat N, Hang DR et al. (2015) Controlling band gap and refractive index in dopant-free α-Fe2O3 films. Electron Mater Lett 11:13–23. https://doi.org/10.1007/s13391-014-4002-0
Uchida M, Flenniken ML, Allen M et al. (2006) Targeting of cancer cells with ferrimagnetic ferritin cage nanoparticles. J Am Chem Soc 128:16626–16633. https://doi.org/10.1021/ja0655690
Bhowmik RN, Mitra P, Choudhury RJ, Reddy VR (2020) Substrate effect on the structural phase formation and magnetic properties of α-Fe2O3 and Ti doped α-Fe2O3 thin films. Appl Surf Sci 501:144224. https://doi.org/10.1016/j.apsusc.2019.144224
Lu Y, Yin Y, Mayers BT, Xia Y (2002) Modifying the surface properties of superparamagnetic iron oxide nanoparticles through a sol-gel approach. Nano Lett 2:183–186. https://doi.org/10.1021/nl015681q
Gupta AK, Gupta M (2005) Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials 26:3995–4021. https://doi.org/10.1016/j.biomaterials.2004.10.012
Jutz G, Van Rijn P, Santos Miranda B, Böker A (2015) Ferritin: a versatile building block for bionanotechnology. Chem Rev 115:1653–1701. https://doi.org/10.1021/cr400011b
Volatron J, Carn F, Kolosnjaj-Tabi J et al. (2017) Ferritin protein regulates the degradation of iron oxide nanoparticles. Small 13:1–13. https://doi.org/10.1002/smll.201602030
He D, Marles-Wright J (2015) Ferritin family proteins and their use in bionanotechnology. N Biotechnol 32:651–657. https://doi.org/10.1016/j.nbt.2014.12.006
Choi WJ, Yano K, Cha M, et al. (2022) Chiral phonons in microcrystals and nanofibrils of biomolecules. Nat Photon. https://doi.org/10.1038/s41566-022-00969-1
Xing X, Chen M, Gong Y et al. (2020) Building memory devices from biocomposite electronic materials. Sci Technol Adv Mater 21:100–121. https://doi.org/10.1080/14686996.2020.1725395
Lv Z, Zhou Y, Han ST, Roy VAL (2018) From biomaterial-based data storage to bio-inspired artificial synapse. Mater Today 21:537–552. https://doi.org/10.1016/j.mattod.2017.12.001
Cobo I, Li M, Sumerlin BS, Perrier S (2015) Smart hybrid materials by conjugation of responsive polymers to biomacromolecules. Nat Mater 14:143–149. https://doi.org/10.1038/nmat4106
Raeis-Hosseini N, Lee JS (2017) Resistive switching memory using biomaterials. J Electroceram 39:223–238. https://doi.org/10.1007/s10832-017-0104-z
Singh A, Konovalov O, Novak J, Vorobiev A (2010) The sequential growth mechanism of a protein monolayer at the air-water interface. Soft Matter 6:3826–3831. https://doi.org/10.1039/b925365c
Singh A, Konovalov O (2013) Measuring elastic properties of a protein monolayer at water surface by lateral compression. Soft Matter 9:2845–2851. https://doi.org/10.1039/c2sm26410b
Hench LL, West JK (1990) The sol-gel process. Chem Rev 90:33–72. https://doi.org/10.1021/cr00099a003
Alagiri M, Hamid SBA (2015) Sol–gel synthesis of α-Fe2O3 nanoparticles and its photocatalytic application. J Sol-Gel Sci Technol 74:783–789
Aydin C, Mansour SA, Alahmed ZA, Yakuphanoglu F (2012) Structural and optical characterization of sol-gel derived boron doped Fe2O3 nanostructured films. J Sol-Gel Sci Technol 62:397–403. https://doi.org/10.1007/s10971-012-2740-8
Mohanty A, Parida A, Raut RK, Behera RK (2022) Ferritin: a promising nanoreactor and nanocarrier for bionanotechnology. ACS Bio Med Chem Au. https://doi.org/10.1021/acsbiomedchemau.2c00003
Xia C, Jia Y, Tao M, Zhang Q (2013) Tuning the band gap of hematite α-Fe2O3 by sulfur doping. Phys Lett Sect A: Gen At Solid State Phys 377:1943–1947
MacHala L, Zboril R, Gedanken A (2007) Amorphous iron (III) oxide—a review. J Phys Chem B 111:4003–4018. https://doi.org/10.1021/jp064992s
Kim CY (2020) Atomic structure of hematite (α-Fe2O3) nanocube surface; synchrotron X-ray diffraction study. Nano-Struct Nano-Objects 23:100497. https://doi.org/10.1016/j.nanoso.2020.100497
Cao D, Li H, Pan L et al. (2016) High saturation magnetization of γ 3-Fe2O3 nano-particles by a facile one-step synthesis approach. Sci Rep 6:1–9. https://doi.org/10.1038/srep32360
Wu J, Mao S, Ye ZG et al. (2010) Room-temperature weak ferromagnetism induced by point defects in α-Fe2O3. ACS Appl Mater Interfaces 2:1561–1564. https://doi.org/10.1021/am1002052
Bhavani P, Rajababu CH, Arif MD et al. (2017) Synthesis of high saturation magnetic iron oxide nanomaterials via low temperature hydrothermal method. J Magn Magn Mater 426:459–466. https://doi.org/10.1016/j.jmmm.2016.09.049
Kodama RH, Berkowitz AE, McNiff EJJ, Foner S (1996) Surface spin disorder in NiFe2O4 nanoparticles. Phys Rev Lett 77:394–397
Phu ND, Ngo DT, Hoang LH, et al. (2011) Crystallization process and magnetic properties of amorphous iron oxide nanoparticles. J Phys D Appl Phys 44. https://doi.org/10.1088/0022-3727/44/34/345002
Silva MF, De Oliveira LAS, Ciciliati MA, et al. (2013) Nanometric particle size and phase controlled synthesis and characterization of γ-Fe2O3 or (α + γ)-Fe2O3 by a modified sol-gel method. J Appl Phys 114. https://doi.org/10.1063/1.4821253
Bhowmik RN, Saravanan A (2010) Surface magnetism, Morin transition, and magnetic dynamics in antiferromagnetic α-Fe2O3 (hematite) nanograins. J Appl Phys 107. https://doi.org/10.1063/1.3327433
Chakrabarty S, Jana TK, De K, et al. (2014) Morphology dependent magnetic properties of α-Fe2O3 nanostructures. Mater Res Express 1. https://doi.org/10.1088/2053-1591/1/4/046104
Biju CS, Raja DH, Padiyan DP (2014) Glycine assisted hydrothermal synthesis of α-Fe2O3 nanoparticles and its size dependent properties. Chem Phys Lett 610–611:103–107. https://doi.org/10.1016/j.cplett.2014.07.024
Sherman DM, Waite TD (1985) Electronic spectra of Fe3+ oxides and oxide hydroxides in the near IR to near UV. Am Mineralogist 70:1262–1269
Wheeler DA, Wang G, Ling Y et al. (2012) Nanostructured hematite: synthesis, characterization, charge carrier dynamics, and photoelectrochemical properties. Energy Environ Sci 5:6682–6702. https://doi.org/10.1039/c2ee00001f
Huheey JE, Keiter EA, Keiter RL (2000) Inorganic chemistry: principles of structure and reactivity, 4th edn. Pearson Education Asia Pte. Ltd., New York
Tauc J, Grigorovici R, Vancu A (1966) Optical properties and electronic structure of amorphous germanium. Phys Status Solidi 15:627–637. https://doi.org/10.1007/978-1-4757-1123-3_5
Mallick P, Dash BN (2013) X-ray diffraction and UV-visible characterizations of α-Fe2O3 nanoparticles annealed at different temperature. Nanosci Nanotechnol 3:130–134. https://doi.org/10.5923/j.nn.20130305.04
Seo O, Tayal A, Kim J et al. (2019) Tuning of structural, optical band gap, and electrical properties of room-temperature-grown epitaxial thin films through the Fe2O3:NiO ratio. Sci Rep 9:1–9. https://doi.org/10.1038/s41598-019-41049-9
Choi S, Lefèvre C, Roulland F et al. (2012) Optical transitions in magnetoelectric Ga0.6Fe1.4O3 from 0.73 to 6.45 eV. J Vac Sci Technol B 30:41204. https://doi.org/10.1116/1.4721649
Acknowledgements
SK thankfully acknowledges the University Grants Commission for National Fellowship scheme for SC (erstwhile–RGNF, Lett. No. F1-17.1/2016-17/RGNF-2015-17-SC-HIM-18502/(SA-III/Website)) for the financial support as fellowship toward pursuance of Ph.D. We acknowledge the UV–VIS facility established at the Himachal Pradesh University, Shimla. We also acknowledge the AMRC, IIT Mandi for SEM facility (JFEI, Nova Nano SEM-450).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kumar, S., Kumar, M., Velaga, S. et al. Tuneable optical properties of Fe2O3 magnetic nanoparticles synthesized from Ferritin. J Sol-Gel Sci Technol 105, 650–661 (2023). https://doi.org/10.1007/s10971-022-05992-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-022-05992-w