Abstract
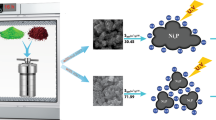
High surface area nickel oxide particles were prepared from thermal decomposition of nanostructured nickel oxalate precursors. The latter were synthesized via a fast and facile method from the reaction between aqueous solutions of nickel chloride and ammonium oxalate in the presence of a surfactant. The effect of several parameters, including surfactant type (PVP, SDS), surfactant concentration (0, 5, 10 g L−1), solution pH (2, 6), and reaction temperature (20, 40, 60 °C) were investigated. The produced particles were characterized by performing X-ray powder diffraction (XRD), scanning electron microscopy (SEM), TG-DTA analysis and Brunauer–Emmett–Teller (BET) adsorption analysis. The synthesized nickel oxalate particles possessed a dominant morphology of cubic or rounded cubic shape varying in size. However, a distinctive flower-shaped structure was obtained in the case of using 5 g L−1 SDS and reaction temperature of 20 °C, which was attributed to the formation of SDS supermicelles and its template function. The 3D structure of the latter consisted of a self-assembly of several petal-like nanosheets with a mean thickness of 16-24 nm. Upon calcination in the air atmosphere, mesoporous nickel oxide particles of the same morphology were obtained with thinner petals (10–22 nm) and specific surface area of 189 m2 g−1. In addition, the photocatalytic activity of the produced NiO nanoparticles against decomposition of Rhodamine B (RhB) pigments under UV irradiation was established and characterized.

Graphical abstract
Highlights
-
Homogeneous precipitation of nickel oxalate nanoparticle precursor from aqueous solution.
-
Solution temperature, solution pH, surfactant type and surfactant concentration was investigated.
-
Obtaining exceptional flower-shaped particle morphology at 20 °C, pH = 6 and 5 g L−1 SDS.
-
High surface area flower-shaped NiO nanoparticles by thermal decomposition of oxalate precursor.
-
Highly efficient photocatalytic activity of NiO nanoparticles towards RhB degradation.












Similar content being viewed by others
References
Jeevanandam J, Barhoum A, Chan YS, Dufresne A, Danquah MK (2018) Review on nanoparticles and nanostructured materials: history, sources, toxicity and regulations. Beilstein J Nanotechnol 9:1050–1074. https://doi.org/10.3762/bjnano.9.98
Wang X, Li L, Zhang YG, Wang S, Zhang Z, Fei L, Qian Y (2006) High-yield synthesis of NiO nanoplatelets and their excellent electrochemical performance. Cryst Growth Des 6:2163–2165. https://doi.org/10.1021/cg060156w
Lim HH, Horri BA, Salamatinia B (2018) Synthesis and Characterizations of Nickel (II) Oxide Sub-Micro Rods via co-precipitation Methods. IOP Confer Ser Mater Sci Eng 398. https://doi.org/10.1088/1757-899X/398/1/012033.
Rakshit S, Ghosh S, Chall S, Mati SS, Moulik SP, Bhattacharya SC (2013) Controlled synthesis of spin glass nickel oxide nanoparticles and evaluation of their potential antimicrobial activity: a cost effective and ecofriendly approach. RSC Adv 3:19348–19356. https://doi.org/10.1039/c3ra42628a
Zhang L, An L, Liu B, Yang H (2011) Synthesis and photocatalytic activity of porous polycrystalline NiO nanowires. Appl Phys A Mater Sci Process 104:69–75. https://doi.org/10.1007/s00339-011-6403-3
Manibalan G, Murugadoss G, Kuppusami P, Kandhasamy N, Rajesh Kumar M (2021) Synthesis of heterogeneous NiO nanoparticles for high-performance electrochemical supercapacitor application. J Mater Sci Mater Electron 32:5945–5954. https://doi.org/10.1007/s10854-021-05315-9
Wang X, Song J, Gao L, Jin J, Zheng H (2005) Optical and electrochemical properties of nanosized NiO via thermal decomposition of nickel oxalate nanofibres. 37:1–4. https://doi.org/10.1088/0957-4484/16/1/009
Fereshteh Z, Salavati-Niasari M, Saberyan K, Hosseinpour-Mashkani SM, Tavakoli F (2012) Synthesis of nickel oxide nanoparticles from thermal decomposition of a new precursor. J Clust Sci 23:577–583. https://doi.org/10.1007/s10876-012-0477-8
Salavati-Niasari M, Mir N, Davar F (2010) A novel precursor in preparation and characterization of nickel oxide nanoparticles via thermal decomposition approach. J Alloy Compd 493:163–168. https://doi.org/10.1016/j.jallcom.2009.11.153
Sattarahmady N, Heli H, Dehdari Vais R (2014) A flower-like nickel oxide nanostructure: Synthesis and application for choline sensing. Talanta 119:207–213. https://doi.org/10.1016/j.talanta.2013.11.002
Lin Y, Xie T, Cheng B, Geng B, Zhang L (2003) Ordered nickel oxide nanowire arrays and their optical absorption properties. Chem Phys Lett 380:521–525. https://doi.org/10.1016/j.cplett.2003.09.066
Sun W, Xiao L, Wu X (2019) Facile synthesis of NiO nanocubes for photocatalysts and supercapacitor electrodes. J Alloy Compd 772:465–471. https://doi.org/10.1016/j.jallcom.2018.09.185
Kavitha T, Yuvaraj H (2011) A facile approach to the synthesis of high-quality NiO nanorods: Electrochemical and antibacterial properties. J Mater Chem 21:15686–15691. https://doi.org/10.1039/c1jm13278d
Wang D, Wang Q, Wang T (2013) Controlled synthesis of porous nickel oxide nanostructures and their electrochemical capacitive behaviors. 559–570. https://doi.org/10.1007/s11581-012-0781-1
Vaidya S, Rastogi P, Agarwal S, Gupta SK, Ahmad T, Antonelli AM, Ramanujachary KV, Lofland SE, Ganguli AK (2008) Nanospheres, nanocubes, and nanorods of nickel oxalate: control of shape and size by surfactant and solvent. J Phys Chem C 112:12610–12615. https://doi.org/10.1021/jp803575h
Rakshit S, Chall S, Mati SS, Roychowdhury A, Moulik SP, Bhattacharya SC (2013) Morphology control of nickel oxalate by soft chemistry and conversion to nickel oxide for application in photocatalysis. RSC Adv 3:6106–6116. https://doi.org/10.1039/c3ra21978j
Jia Z, Liu J, Wang Q, Ye M, Zhu R (2014) Facile preparation of mesoporous nickel oxide microspheres and their adsorption property for methyl orange from aqueous solution. Mater Sci Semicond Process 26:716–725. https://doi.org/10.1016/j.mssp.2014.06.026
Sathishkumar K, Shanmugam N, Kannadasan N, Cholan S, Viruthagiri G (2014) Synthesis and characterization of nanocrystalline nickel oxide using NaOH and oxalic acid as oxide sources. Mater Res Express 1:026104. https://doi.org/10.1088/2053-1591/1/2/026104
Alagiri M, Ponnusamy S, Muthamizhchelvan C (2012) Synthesis and characterization of NiO nanoparticles by sol-gel method. J Mater Sci Mater Electron 23:728–732. https://doi.org/10.1007/s10854-011-0479-6
Wu Y, He Y, Wu T, Chen T, Weng W, Wan H (2007) Influence of some parameters on the synthesis of nanosized NiO material by modified sol-gel method. Mater Lett 61:3174–3178. https://doi.org/10.1016/j.matlet.2006.11.018
Bose P, Ghosh S, Basak S, Naskar MK (2016) A facile synthesis of mesoporous NiO nanosheets and their application in CO oxidation. J Asian Ceram Soc 4:1–5. https://doi.org/10.1016/j.jascer.2016.01.006
Goel S, Tomar AK, Sharma RK, Singh G (2018) Highly pseudocapacitive NiO nanoflakes through surfactant-free facile microwave-assisted route. ACS Appl Energy Mater 1:1540–1548. https://doi.org/10.1021/acsaem.7b00343
Suresh R, Ponnuswamy V, Sankar C, Manickam M, Venkatesan S, Perumal S (2017) NiO nanoflakes: effect of anions on the structural, optical, morphological and magnetic properties. J Magn Magn Mater 441:787–794. https://doi.org/10.1016/j.jmmm.2017.05.069
Babu GA, Navaneethan GRM (2014) An investigation of flower shaped NiO nanostructures by microwave and hydrothermal route. 5231–5240. https://doi.org/10.1007/s10854-014-2293-4
Cao S, Han T, Peng L (2020) Surfactant-free synthesis of 3D hierarchical flower-like NiO nanostructures with enhanced ethanol-sensing performance. J Mater Sci Mater Electron. https://doi.org/10.1007/s10854-020-04283-w
Wang M, Zhu Y, Han L, Qi R, He F (2019) Inky flower-like supermicelles assembled from π-conjugated block copolymers. Polym Chem 11:61–67. https://doi.org/10.1039/c9py01625b
Shende P, Kasture P, Gaud RS (2018) Nanoflowers: the future trend of nanotechnology for multi-applications. Artif Cells Nanomed Biotechnol 46:S413–S422. https://doi.org/10.1080/21691401.2018.1428812
Wu Q, Liu Y, Hu Z (2013) Flower-like NiO microspheres prepared by facile method as supercapacitor electrodes. J Solid State Electrochem 17:1711–1716. https://doi.org/10.1007/s10008-013-2022-6
Wang J, Zeng W, Wang Z (2015) Assembly of 2D nanosheets into 3D flower-like NiO: Synthesis and the in fl uence of petal thickness on gas-sensing properties. https://doi.org/10.1016/j.ceramint.2015.11.150
Gao Q, Zeng W, Miao R (2016) Synthesis of multifarious hierarchical flower-like NiO and their gas-sensing properties. J Mater Sci Mater Electron 27:9410–9416. https://doi.org/10.1007/s10854-016-4986-3
Luo L, Wu Y, Wei F, Shi J, Cheng L (2010) Synthesis and characterterization of flower-like NiO nano-architectures by homogeneous precipitation. Key Eng Mater 434–435:554–557
Zhao B, Ke XK, Bao JH, Wang CL, Dong L, Chen YW, Chen HL (2009) Synthesis of flower-like NiO and effects of morphology on its catalytic properties. J Phys Chem C 113:14440–14447. https://doi.org/10.1021/jp904186k
Mahmoudi S, Jafari A, Javadian S (2019) Temperature effect on performance of nanoparticle/surfactant flooding in enhanced heavy oil recovery. Pet Sci 16:1387–1402. https://doi.org/10.1007/s12182-019-00364-6
Pakuro N, Yakimansky A, Chibirova F, Arest-Yakubovich A (2009) Thermo- and pH-sensitivity of aqueous poly(N-vinylpyrrolidone) solutions in the presence of organic acids. Polymer 50:148–153. https://doi.org/10.1016/j.polymer.2008.10.037
Liu Z, Xu K, Sun H, Yin S (2015) One-step synthesis of single-layer MnO2 nanosheets with multi-role sodium dodecyl sulfate for high-performance pseudocapacitors. Small 11:2182–2191. https://doi.org/10.1002/smll.201402222
Charboneau J, Von Wandruszka R (2010) The clouding of an anionic surfactant in acid solution: Mechanistic and analytical implications. J Surfactants Deterg 13:281–286. https://doi.org/10.1007/s11743-009-1174-y
Okamoto T, Yang JG, Kuroda K, Ichino R, Okido M (2007) Preparation of size and aggregation controlled nickel oxalate dihydrate particles from nickel hydroxide. Adv Mater Res 15–17:581–586
Behnoudnia F, Dehghani H (2014) Anion effect on the control of morphology for NiC2O4·2H2O nanostructures as precursors for synthesis of Ni(OH)2 and NiO nanostructures and their application for removing heavy metal ions of cadmium (II) and lead (II). Dalton Trans 43:3471–3478. https://doi.org/10.1039/C3DT52049H
Xue X, Penn RL, Leite ER, Huang F, Lin Z (2014) Crystal growth by oriented attachment: Kinetic models and control factors. Cryst Eng Comm 16:1419–1429. https://doi.org/10.1039/c3ce42129e
Madras G, McCoy BJ (2003) Temperature effects during Ostwald ripening. J Chem Phys 119:1683–1693. https://doi.org/10.1063/1.1578617
Dehghan Noudeh GH, Housaindokht M, Fazly Bazzaz BS (2007) The effect of temperature on thermodynamic parameters of micellization of some surfactants. J Appl Sci 7:47–52
Li X, Gao Y, Boott CE, Winnik MA, Manners I (2015) Non-covalent synthesis of supermicelles with complex architectures using spatially confined hydrogen-bonding interactions. Nat Commun 6:1–8. https://doi.org/10.1038/ncomms9127
Gould OEC, Qiu H, Lunn DJ, Rowden J, Harniman RL, Hudson ZM, Winnik MA, Miles MJ, Manners I (2015) Transformation and patterning of supermicelles using dynamic holographic assembly. Nat Commun 6:1–7. https://doi.org/10.1038/ncomms10009
Kulthe SS, Choudhari YM, Inamdar NN, Mourya V (2012) Polymeric micelles: Authoritative aspects for drug delivery. Des Monomers Polym 15:465–521. https://doi.org/10.1080/1385772X.2012.688328
Xu J, Fang Y, Xia Y, Wang C, He M (2005) Preparation of nickel nanoparticles in the presence of sodium dodecyl sulfate polyvinylpyrrolidone clusters. AIChE Annu Meet Confer Proc 1430–1435.
Samiey B, Cheng CH, Wu J (2014) Effects of surfactants on the rate of chemical reactions. J Chem 2014. https://doi.org/10.1155/2014/908476
Thota S, Kumar J (2007) Sol–gel synthesis and anomalous magnetic behaviour of NiO nanoparticles. J Phys Chem Solids 68:1951–1964. https://doi.org/10.1016/j.jpcs.2007.06.010
Thommes M (2010) Physical adsorption characterization of nanoporous materials. Chem-Ing-Tech 82:1059–1073. https://doi.org/10.1002/cite.201000064
Kazeminezhad I, Sadollahkhani A (2016) Influence of pH on the photocatalytic activity of ZnO nanoparticles. J Mater Sci Mater Electron 27:4206–4215. https://doi.org/10.1007/s10854-016-4284-0
Wan X, Yuan M, Tie SL, Lan S (2013) Effects of catalyst characters on the photocatalytic activity and process of NiO nanoparticles in the degradation of methylene blue. Appl Surf Sci 277:40–46. https://doi.org/10.1016/j.apsusc.2013.03.126
Abboud M, Mubarak AT, Hamdy MS, Abu Haija M, Ismail I, Bel-Hadj-Tahar R, Bel-Hadj-Tahar R (2020) Highly ordered mesoporous flower-like NiO nanoparticles: Synthesis, characterization and photocatalytic performance. N J Chem 44:3402–3411. https://doi.org/10.1039/c9nj04955j
Kalaie MR, Youzbashi AA, Meshkot MA, Hosseini-Nasab F (2016) Preparation and characterization of superparamagnetic nickel oxide particles by chemical route. Appl Nanosci 6:789–795. https://doi.org/10.1007/s13204-015-0498-3
Al-Sehemi AG, Al-Shihri AS, Kalam A, Dud G, Ahmad T (2014) Microwave synthesis, optical properties and surface area studies of NiO nanoparticles. J Mol Struct 1058:56–61. https://doi.org/10.1016/j.molstruc.2013.10.065
Marei NN, Nassar NN, Vitale G (2016) The effect of the nanosize on surface properties of NiO nanoparticles for the adsorption of Quinolin-65. Phys Chem Chem Phys 18:6839–6849. https://doi.org/10.1039/c6cp00001k
Nguyen K, Hoa ND, Hung CM, Thanh Le DT, Van Duy N, Van Hieu N (2018) A comparative study on the electrochemical properties of nanoporous nickel oxide nanowires and nanosheets prepared by a hydrothermal method. RSC Adv 8:19449–19455. https://doi.org/10.1039/c8ra02862a
Ren Y, Gao L (2010) From three-dimensional flower-like α-Ni(OH)2 nanostructures to hierarchical porous NiO nanoflowers: Microwave-assisted fabrication and supercapacitor properties. J Am Ceram Soc 93:3560–3564. https://doi.org/10.1111/j.1551-2916.2010.04090.x
Sharma RK, Ghose R (2015) Superlattices and microstructures synthesis of porous nanocrystalline NiO with hexagonal sheet-like morphology by homogeneous precipitation method. Superlattices Microstruct 80:169–180. https://doi.org/10.1016/j.spmi.2014.12.034
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. PZ performed data collection and analysis, and wrote the first draft of the manuscript. All authors commented on previous versions of the manuscript and read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zahabi, P., Zakeri, A. & Asadrokht, M. A facile surfactant-assisted precipitation route to self-assembled 3D flower-shaped NiO nanostructures with enhanced surface area. J Sol-Gel Sci Technol 105, 73–84 (2023). https://doi.org/10.1007/s10971-022-05980-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-022-05980-0