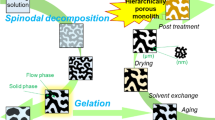
Abstract
A bimodal amorphous silica-alumina with macropores and mesopores of sizes 2–4 µm and 10 nm, respectively, was prepared with an alumina content of up to 30 wt% using a sol–gel method. The requisite amount of water to generate the 2–4 µm macropores decreased with increasing alumina content. However, this decrease reached a limiting value, and the gelation temperature needed to be increased at alumina contents ≥20 wt%. Because the alkaline resistance increases with increasing alumina content, the aging temperature needed to be increased to obtain 10 nm mesopores. In the acidic isomerization of 1-octene over silica-alumina, homogeneous sol–gel samples were more active than those heterogeneously impregnated. The conversion of 1-octene increased with increasing alumina content up to 15 wt%, while the activity decreased above 30 wt% because of because of Al2O3 aggregation. The catalytic results, together with the temperature-programmed desorption of NH3, indicate that the sol–gel sample generates Brønsted acid sites via the formation of Si-O-Al bonds, whereas the impregnated sample probably generates Lewis acid sites.

Graphical abstract
Highlights
-
Meso-macro bimodal amorphous silica-alumina with a mesopore size of 10 nm was prepared using a sol–gel method.
-
The morphology changed with increasing content of water and alumina.
-
The sol–gel sample showed an improved 1-octene conversion compared to the impregnated sample at all alumina contents.
-
The sol–gel sample mainly generated Brønsted acid sites via the formation of Si-O-Al bonds.
-
The impregnated sample probably generated Lewis sites owing to the aggregation of alumina on the surface.









Similar content being viewed by others
References
Takahashi R, Sato S, Sodesawa T, Arai K, Yabuki M (2005) Effect of diffusion in catalytic dehydration of alcohol over silica–alumina with continuous macropores. J Catal 229:24–29
Nakanishi K, Komura H, Takahashi R, Soga N (1994) Phase Separation in Silica Sol–Gel System Containing Poly(ethylene oxide). I. Phase Relation and Gel Morphology. Bull Chem Soc Jpn 67:1327–1335
Anne G, Zakaria A, Bilel S, Youcef D, Katarzyna S, Andrzej J, Franck T, Hadj H, Abdelkader B, Francesco R, Francois F (2016) Synthesis and Textural Characterization of Mesoporous and Meso-/Macroporous Silica Monoliths Obtained by Spinodal Decomposition. Inorganics 4:9
Mingliang S, Tianbo Z, Zhaofei M, Zunfeng L (2019) Facile preparation of macro-mesoporous zirconium titanate monoliths via a sol–gel reaction accompanied by phase separation. J Mater Res 34:4066–4075
Takahashi R, Sato S, Sodesawa T, Yabuki M (2001) Silica–Alumina Catalyst with Bimodal Pore Structure Prepared by Phase Separation in Sol–Gel Process. J Catal 200:197–202
Yabuki M, Takahashi R, Sato S, Sodesawa T, Ogura K (2002) Silica–alumina catalysts prepared in sol–gel process of TEOS with organic additives. Phys Chem Chem Phys 4:4830–4837
Takahashi R, Sato S, Tomiyama S, Ohashi T, Nakamura N (2007) Pore structure control in Ni/SiO2 catalysts with both macropores and mesopores. Microporous Mesoporous Mater 98:107–114
Ishihara A, Negura H, Hashimoto T, Nasu H (2010) Catalytic properties of amorphous silica-alumina prepared using malic acid as a matrix in catalytic cracking of n-dodecane. Appl Catal A: Gen 388:68–76
Sarazen M, Doskocil E, Iglesia E (2016) Catalysis on solid acids: Mechanism and catalyst descriptors in oligomerization reactions of light alkenes. J Catal 344:553–569
Ohkita H, Nishiyama R, Tochihara Y, Mizushima T, Kakuta N, Morioka Y, Ueno A, Namiki Y, Tanifuji S, Katoh H, Sunazuka H, Nakayama R, Kuroyanagi T (1993) Acid properties of silica-alumina catalysts and catalytic degradation of polyethylene. Ind Eng Chem Res 32:3112–3116
Kim Y, Jung K, Park E (2011) Gas-phase dehydration of glycerol over silica–alumina catalysts. Appl Catal B: Environ 107:177–187
Kumbhalkar M, Buchanan S, Huber G, Dumesic J (2017) Ring Opening of Biomass-Derived Cyclic Ethers to Dienes over Silica/Alumina. ACS Catal 7:5248–5256
Segawa A, Ichijo T, Kimura N, Tsuruta K, Yoshida N, Okamoto M (2020) 1,3-Butadiene Production by Crotyl Alcohol Dehydration over Solid Acids and Catalyst Deactivation by Water Adsorption. J Jpn Petro Inst 63:70–78
Matsumoto Y, Mita L, Hashimoto K, Tokoroyama T (1995) Preparation of silica-alumina catalyst by the sol-gel method and its activity for the Diels-Alder reaction of isoprene and acrylaldehyde. Appl Catal A: Gen 131:L1–L6
Marczewski M, Kominiak M, Dul M, Marczewska H (2016) The role of butylbenzene carbenium ions in the acid catalyzed cracking of polystyrene. Transformation of n-butylbenzene, sec-butylbenzene, iso-butylbenzene, tert-butylbenzene, 4-phenyl-1-butene, n-propylbenzene and n-hexylbenzene over silicaalumina and alumina acid catalysts React Kinet Mech. Cat 119:107–120
Xu L, Bao X, Lv M, Duan L, Liu Z (2014) Effect of Aluminium Content on the Structural, Acidic and Catalytic Properties of Mesoporous Aluminosilicate Materials. Asian J Chem 26:7315–7317
Xu L, Liu Z, Li Z, Liu J, Ma Y, Guan J, Kan Q (2011) Non-crystalline mesoporous aluminosilicates catalysts: Synthesis, characterization and catalytic applications. J Non-Cryst Solid 357:1335–1341
Tanabe K., Misono M., Ono Y. (1989) NEW SOLID ACIDS AND BASES their catalytic properties. Kodansha, Tokyo
Sato S, Morimoto Y, Takatsuka T, Hashimoto H (1986) Matrix Pore Structure and Bottom Cracking Capability of Residue FCC Catalysts. J Jpn Pet Inst 29:60–65
Larabi C, Norsic S, Khrouz L, Boyron O, Szeto K, Lucas C, Taoufik M, Mallmann A (2020) Oxide-Supported Titanium Catalysts: Structure–Activity Relationship in Heterogeneous Catalysis, with the Choice of Support as a Key Step. Organometallics 39:4608–4617
Jeon M, Mutairi A, Jung H, Hong I, An J, Park C, Kim D, Jeon Y, Marafi A, Ma X, Park J (2020) Molecular Characteristics of Light Cycle Oil Hydrodesulfurization over Silica–Alumina-Supported NiMo Catalysts. ACS Omega 5:29746–29754
Kubicek N, Vaudry F, Chiche B, Hudec P, Renzo F, Schulz P, Fajula F (1998) Stabilization of zeolite beta for fcc application by embedding in amorphous matrix. Appl Catal A: Gen 175:159–171
Corma A, Martinez A, Pergher S, Peratello S, Perego C, Bellusi G (1997) Global overview of research in catalysis Norway. Appl Catal A: Gen 152:107–125
Rajagopalan K, Habib E (1992) Understand FCC matrix technology. Hydrocarbon Process 71:43
Climent M, Corma A, Iborra S, Navarro M, Primo J (1996) Use of Mesoporous MCM-41 Aluminosilicates as Catalysts in the Production of Fine Chemicals: Preparation of Dimethylacetals. J Catal 161:783–78
Ishihara A, Nakajima K, Hirado M, Hashimoto T, Nasu H (2011) Novel Method for Generating Large Mesopores in an Amorphous Silica–Alumina by Controlling the Pore Size with the Gel Skeletal Reinforcement and Its Catalytic Cracking Properties as a Catalyst Matrix. Chem Lett 40:558–560
Tanabe K, Ishiya C, Matsuzaki I, Ichikawa I, Hattori H (1972) Acidic Property and Catalytic Activity of TiO2·ZnO. Bull Chem Soc Jpn 45:47–51
Brunauer S, Emmett P, Teller E (1938) Adsorption of Gases in Multimolecular Layers. J Am Chem Soc 60:309–319
Dollomore D, Heal G (1964) An improved method for the calculation of pore size distribution from adsorption data. J Appl Chem 14:109–114
Takahashi R, Sato S, Sodesawa T, Suzuki K, Matsumoto T, Nakanishi K, Soga N (2001) Phase Separation in Sol–Gel Process of Alkoxide-Derived Silica-Zirconia in the Presence of Polyethylene Oxide. J Am Ceram Soc 84:1968–1976
Nakanishi K (1997) Pore Structure Control of Silica Gels Based on Phase Separation. J Porous Mater 4:67–112
Nakamura N, Takahashi R, Sato S, Sodesawa T, Yoshida S (2000) Ni/SiO2 catalyst with hierarchical pore structure prepared by phase separation in sol–gel process. Phys Chem Chem Phys 2:4983–4990
Takahashi R, Sato S, Sodesawa T, Nakamura N, Tomiyama S, Kosugi T, Yoshida S (2001) Nanosize Ni/SiO2 Catalyst Prepared by Homogeneous Precipitation in Wet Silica Gel. J Nanosci Nanotechnol 1:169–176
Takahashi R, Sato S, Sodesawa T, Shizukuishi M, Morofuji K, Ogura K (2005) Change in local coordination structure of aluminum cations in silica–alumina solution during coprecipitation. J Non-Cryst Solids 351:826–832
Acknowledgements
The SEM observation was conducted at the Advanced Research Support Center, Ehime University.
Author information
Authors and Affiliations
Contributions
MI performed most of the experimental steps, such as sample preparation, catalytic tests, TPD, and N2 adsorption, summarized the data, and wrote the first draft. FS designed the study and edited the paper. RT supervised this study from the perspective of inorganic chemistry.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Inoue, M., Takahashi, R. & Sato, F. Preparation of meso-macro bimodal porous amorphous silica-alumina as an acidic catalyst. J Sol-Gel Sci Technol 104, 617–626 (2022). https://doi.org/10.1007/s10971-022-05969-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-022-05969-9