Abstract
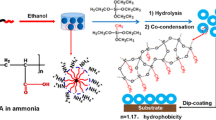
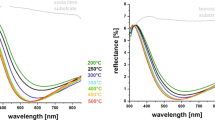
In this study, we investigate the prospect of using dual alkoxysilane precursor chemistry for the scalable deposition of antireflection thin films on soda lime silicate glass. The hybrid chemistry involves the use of tetraethyl orthosilicate (TEOS) in combination with methyltriethoxysilane (MTES). Throughout the study, we use a dual acidic catalyst system comprising an organic (acetic acid) and an inorganic (nitric acid) acid. After the coating process, the coated glasses are cured at 100 °C and then annealed at 700 °C to mimic a typical industrial tempering process. The effects of altering the MTES/TEOS mole ratio on the resulting colloidal sol are studied with five different sols using Fourier Transform Infrared (FTIR) analysis and the final properties of solid thin films are investigated in detail through optical spectrophotometry, contact angle measurements, optical microscopy and electron microscopy (Scanning Electron Microscopy (SEM) and Scanning Transmission Electron Microscopy (STEM)) techniques. In addition to the coated glasses, we investigate the thermal stability of dried gels at room conditions as well as at 100 °C, 400 °C, and 700 °C annealing temperatures. Since the PV panel glasses are typically deployed to stay in contact with the external environment, the weathering durability of the samples having optimum properties are investigated in accordance with EN 1096-2 and IEC 61215 standard test methods. It is shown that the presented dual precursor chemistry can produce coatings that exhibit high mechanical and chemical resistance and retain their anti-reflective properties when treated with an industrial tempering process. Finally, we provide evidence that laboratory scale dip coating process can be directly scaled up to a roller coating process without compromising optical performance. Our results show that commercial solar module dimensions and patterned glasses can be directly accommodated with the proposed coating chemistry.

Sample preparation process flow chart and contact angle measurements of coated samples which were cured at 100 °C and then annealed at 700 °C using S0.3, S0.6, S1.2, S2.4, and S4.8 solutions with different time intervals.
Highlights
-
Highly durable monolayer anti-reflective (AR) coatings that can be annealed with an industrial tempering process up to 700 oC are demonstrated.
-
Using a dual precursor chemistry based on tetraethyl orthosilicate (TEOS) and methyltriethoxysilane (MTES) highly durable AR coatings are obtained and are tested according to EN1096-2 and IEC 61215 standards.
-
In the presence of a dual acid catalyst system, changes in MTES/TEOS molar ratio fundamentally affect the durability of the AR coatings.
-
Coating with sols aged up to 7 weeks allows us to demonstrate the long-term stability of the coating chemistry.
-
Using an industrial grade roll coater, we show that the proposed coating chemistry can be directly scaled-up.










Similar content being viewed by others
References
Hecht E (2017) Optics. 5th edn. Pearson Education, Harlow, Essex.
Glaser HJ (2000) Large area glass coating. Von Ardenne Anlagentechnik GMBH.
Chen D (2001) Anti-reflection (AR) coatings made by sol–gel processes: a review. Sol Energy Mater Sol Cells 68(3):313–336.
Glaubitt W, Löbmann P (2012) Antireflective coatings prepared by sol–gel processing: Principles and applications. J Eur Ceram Soc 32(11):2995–2999.
Ganjoo A et al. (2019) Glass and coatings on glass for solar applications. In: Musgraves JD, Hu J, and Calvez L (eds). Springer handbook of glass. Springer International Publishing, Cham. p. 1635–1676.
Yoldas BE (1980) Investigations of porous oxides as an antireflective coating for glass surfaces. Appl Opt 19(9):1425–1429.
Thomas IM (1986) High laser damage threshold porous silica antireflective coating. Appl Opt 25(9):1481–1483.
Thomas IM (1992) Method for the preparation of porous silica antireflection coatings varying in refractive index from 1.22 to 1.44. Appl Opt 31(28):6145–6149.
Xi JQ et al. (2007) Optical thin-film materials with low refractive index for broadband elimination of Fresnel reflection. Nat Photonics 1(3):176–179.
Xi JQ, Kim JK, Schubert EF (2005) Silica nanorod-array films with very low refractive indices. Nano Lett 5(7):1385–1387.
Guo Z et al. (2016) High-quality hollow closed-pore silica antireflection coatings based on styrene–acrylate emulsion @ organic–inorganic silica precursor. ACS Appl Mater Interfaces 8(18):11796–11805.
Du Y et al. (2010) Hollow silica nanoparticles in UV−visible antireflection coatings for poly(methyl methacrylate) substrates. ACS Nano 4(7):4308–4316.
Gao T, Jelle BP, Gustavsen A (2013) Antireflection properties of monodisperse hollow silica nanospheres. Appl Phys A 110(1):65–70.
Nielsen KH et al. (2014) Optical breathing of nano-porous antireflective coatings through adsorption and desorption of water. Sci Rep. 4(1):6595.
Cai S et al. (2014) Sol-gel preparation of hydrophobic silica antireflective coatings with low refractive index by base/acid two-step catalysis. ACS Appl Mater Interfaces 6(14):11470–11475.
Ma Y, Kanezashi M, Tsuru T (2010) Preparation of organic/inorganic hybrid silica using methyltriethoxysilane and tetraethoxysilane as co-precursors. J Sol-Gel Sci Technol 53(1):93–99.
Lin W et al. (2018) Characterization of sol-gel ORMOSIL antireflective coatings from phenyltriethoxysilane and tetraethoxysilane: microstructure control and application. Surf Coat Technol 345:177–182.
Chi F et al. (2020) Aggregation of silica nanoparticles in sol–gel processes to create optical coatings with controllable ultralow refractive indices. ACS Appl Mater Interfaces 12(14):16887–16895.
Muhammad AZ et al. (2021) Improved optical performance of hydrophobic silica nanoparticles as antireflection coating on glass and its electrical performance for photovoltaic module applications. Optical Eng 60(5):1–9.
Yoldas BE (1975) Alumina gels that form porous transparent Al2O3. J Mater Sci 10(11):1856–1860.
Aydin G (2021) Tek katmanlı yansıma önleyici ince film kaplamaların üretimi ve optik/mekanik özelliklerinin incelenemesi. İstanbul Technical University, Turkey.
Whitehouse D (2002) Surfaces and their measurement. Hermes Penton Science, London.
Pai PG et al. (1986) Infrared spectroscopic study of SiOx films produced by plasma enhanced chemical vapor deposition. J Vac Sci Technol A 4(3):689–694.
Morrow BA (1990) Surface groups on oxides. In: Fierro JLG (ed) Studies in surface science and catalysis. Elsevier. p. A161–A224.
Zhao X et al. (2021) The influence of water content on the growth of the hybrid-silica particles by sol-gel method. Silicon 13(10):3413–3421.
Kamiya K et al. (1990) Thermal evolution of gels derived from CH3Si(OC2H5)3 by the sol-gel method. J Non-Crystalline Solids 121(1):182–187.
Yu S et al. (2003) The effect of TEOS/MTES ratio on the structural and dielectric properties of porous silica films. J Electrochem Soc 150(5):F116.
Koga Y et al. (2011) Is a methyl group always hydrophobic? Hydrophilicity of trimethylamine-n-oxide, tetramethyl urea and tetramethylammonium ion. J Phys Chem B 115(12):2995–3002.
Biebuyck HA, Whitesides GM (1994) Self-organization of organic liquids on patterned self-assembled monolayers of alkanethiolates on gold. Langmuir 10(8):2790–2793.
van Gestel MAC, He B, Darhuber AA (2020) Formation of residual droplets upon dip-coating of chemical and topographical surface patterns on partially wettable substrates. Chem Eng Sci 227:115832.
Blake TD, Ruschak KJ (1979) A maximum speed of wetting. Nature 282(5738):489–491.
Brinker CJ Dip coating. In: Schneller T et al. (eds.) Chemical solution deposition of functional oxide thin films. Springer Vienna, Vienna. p. 233–261.
Nečas D, Klapetek P (2012) Gwyddion: an open-source software for SPM data analysis. Cent Eur J Phys 10(1):181–188.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sobacı, G.A., Okan, O.B., Kazmanlı, K. et al. Scalable deposition of sol–gel based monolayer antireflective thin films by using a dual alkoxysilane precursor chemistry. J Sol-Gel Sci Technol 102, 493–503 (2022). https://doi.org/10.1007/s10971-022-05815-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-022-05815-y