Abstract
Silicic acid components from bioactive glass activate osteoblasts gene for bone generation. Many studies have been reported on osteoblast compatibility and bone regeneration using composites and hybrids, including silica or siloxane units. We previously synthesized chitosan−siloxane hybrids via a sol-gel method and observed bone and nerve regeneration. However, it is not clear the structure of molecules involving silicon atoms that has a more effective role in the cell activity and their mechanisms of cell activation. In this study, we prepared hybrid materials from chitosan and different types of alkoxysilane, 3-glycidoxypropyltrimethoxysilane (GPTMS), 3-glycidoxypropyldimethoxymethylsilane (GPDMS), and tetraethoxysilane (TEOS), and investigated the structures of the silicon-containing species dissolved from each hybrid and their effect on the proliferation of nerve cells. The silicon-containing species in the extraction were mainly 100–600 molecular weight, indicating they were chitin/chitosan units and monomeric hydrolyzed GPTMS, GPDMS, and TEOS. The nerve cell proliferation was inhibited by the chitosan–GPTMS and GPDMS hybrid extractions. The silicon-containing species were not taken up by the cells. The silicon-containing species dissolved from the hybrids were adsorbed to the cells or they inactivated biomolecules in the culture medium, suppressing cell proliferation.

Graphical abstract
Highlights
-
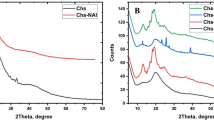
The silicon-containing species dissolved from chitosan–siloxane hybrid were corresponding to hydrolyzed GPTMS, GPDMS, and TEOS with chitosan oligomers.
-
Nerve cells did not uptake the silicon-containing species.
-
The silicon-containing species derived from Chitosan–GPTMS and –GPDMS hybrids inhibited nerve cell proliferation.
-
The silicon-containing species derive the hybrids adsorbed to the cell surface or inactivated biomolecules in the medium.












Similar content being viewed by others
References
Lansdown AB, Williams A (2007) A prospective analysis of the role of silicon in wound care. J Wound Care 16(9):404–407. https://doi.org/10.12968/jowc.2007.16.9.27865
Carlisle EM (1988) Silicon as a trace nutrient. Sci Total Environ 73(1–2):95–106. https://doi.org/10.1016/0048-9697(88)90190-8
Xynos ID, Edgar AJ, Buttery LDK, Hench LL, Polak JM (2000) Ionic products of bioactive glass dissolution increase proliferation of human osteoblasts and induce insulin-like growth factor II mRNA expression and protein synthesis. Biochem Biophys Res Commun 276(2):461–465. https://doi.org/10.1006/bbrc.2000.3503
Gough JE, Jones JR, Hench LL (2004) Nodule formation and mineralisation of human primary osteoblasts cultured on a porous bioactive glass scaffold. Biomaterials 25(11):2039–2046. https://doi.org/10.1016/j.biomaterials.2003.07.001
Shie MY, Ding SJ, Chang HC (2011) The role of silicon in osteoblast-like cell proliferation and apoptosis. Acta Biomater 7(6):2604–2614. https://doi.org/10.1016/j.actbio.2011.02.023
Zhou X, Moussa FM, Mankoci S, Ustriyana P, Zhang N, Abdelmagid S, Molenda J, Murphy WL, Safadi FF, Sahai N (2016) Orthosilicic acid, Si(OH)4, stimulates osteoblast differentiation in vitro by upregulating miR-146a to antagonize NF-κB activation. Acta Biomater 39:192–202. https://doi.org/10.1016/j.actbio.2016.05.007
Kim KJ, Joe YA, Kim MK, Lee SJ, Ryu YH, Cho DW, Rhie JW (2015) Silica nanoparticles increase human adipose tissue-derived stem cell proliferation through ERK 1/2 activation. Int J Nanomed 10:2261–2272. https://doi.org/10.2147/IJN.S71925
Quignard S, Coradin T, Powell JJ, Jugdaohsingh R (2017) Silica nanoparticles as sources of silicic acid favoring wound healing in vitro. Colloids Surf B 155:530–537. https://doi.org/10.1016/j.colsurfb.2017.04.049
Fritsch-Decker S, An Z, Yan J, Hansjosten I, Al-Rawi M, Peravali R, Diabaté S, Weiss C (2019) Silica nanoparticles provoke cell death independent of p53 and BAX in human colon cancer cells. Nanomaterials 9(8):1172. https://doi.org/10.3390/nano9081172
Bonazza V, Borsani E, Buffoli B, Parolini S, Inchingolo F, Rezzani R, Rodella LF (2018) In vitro treatment with concentrated growth factors (CGF) and sodium orthosilicate positively affects cell renewal in three different human cell lines. Cell Biol Int 42(3):353–364. https://doi.org/10.1002/cbin.1090
Shirosaki Y, Tsuru K, Moribayashi H, Hayakawa S, Nakamura Y, Gibson IR, Osaka A (2010) Preparation of osteocompatible Si(IV)-enriched chitosan-silicate hybrids. J Ceram Soc Jpn 119(11):989–992. https://doi.org/10.2109/jcersj2.118.989
Teruo O, Masayuki Y (2007) In: Recent progress in Regenerative Medicine Technologies, Chapter 4 Soft Tissues. Tokyo, Japan
Gupta B, Papke JB, Mohammadkhah A, Day DE, Harkins AB (2016) Effects of chemically doped bioactive borate glass on neuron regrowth and regeneration. Ann Biomed Eng 44(12):3468–3477. https://doi.org/10.1007/s10439-016-1689-0
Mobasseri SA, Terenghi G, Downes S (2013) Micro-structural geometry of thin films intended for the inner lumen of nerve conduits affects nerve repair. J Mater Sci: Mater Med 24(7):1639–1647. https://doi.org/10.1007/s10856-013-4922-5
Jiang X, Lim SH, Mao Hai-Quan HQ, Chew SY (2010) Current applications and future perspectives of artificial nerve conduits. Exp Neurol 223(1):86–101. https://doi.org/10.1016/j.expneurol.2009.09.009
Boecker A, Daeschler SC, Kneser U, Harhaus L (2019) Relevance and recent developments of chitosan in peripheral nerve surgery. Front Cell Neurosci 13:104. https://doi.org/10.3389/fncel.2019.00104
Dietzmeyer N, Förthmann M, Grothe C, Haastert-Talini K (2020) Chitosan tubes prefilled with aligned fibrin nanofiber hydrogel enhance facial nerve regeneration in rabbits. ACS Omega 6:26293–26301. https://doi.org/10.4103/1673-5374.271668
Mu X, Sun X, Yang S, Pan S, Sun J, Niu Y, He L, Wang X (2021) Dog sciatic nerve regeneration across a 30-mm defect bridged by a chitosan/PGA artificial nerve graft. Brain 128(8):1897–1910. https://doi.org/10.1021/acsomega.1c03245
Amado S, Simões MJ, Armada da Silva PAS, Luís AL, Shirosaki Y, Lopes MA, Santos JD, Fregnan F, Gambarotta G, Raimondo S, Fornaro M, Veloso AP, Varejão ASP, Maurício AC, Geuna S (2008) Use of hybrid chitosan membranes and N1E-115 cells for promoting nerve regeneration in an axonotmesis rat model. Biomaterials 29(33):4409–4419. https://doi.org/10.1016/j.biomaterials.2008.07.043
Simões MJ, Amado S, Gärtner A, Armada da Silva PAS, Raimondo S, Vieira M, Luís AL, Shirosaki Y, Veloso AP, Santos JD, Varejão ASP, Geuna S, Maurício AC (2010) Use of chitosan scaffolds for repairing rat sciatic nerve defects. Ital J Anat Embryol 115(3):190–210. https://doi.org/10.13128/IJAE-9074
Simões MJ, Gärtner A, Shirosaki Y, Gil da Costa RM, Cortez PP, Gärtner F, Santos JD, Lopes MA, Veloso AP, Varejão ASP, Maurício AC (2011) In vitro and in vivo chitosan membranes testing for peripheral nerve reconstruction. Acta Med Port 24(1):43–51. https://doi.org/10.20344/AMP.344
Shirosaki Y, Hayakawa S, Osaka A, Lopes MA, Santos JD, Geuna S, Maurício AC (2014) Challenging for nerve repair using chitosan-siloxane hybrid porous scaffolds. BioMed Res Int 153808. https://doi.org/10.1155/2014/153808
Berridge MV, Tan AS (1993) Characterization of the cellular reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT): subcellular localization, substrate dependence, and involvement of mitochondrial electron transport in MTT reduction. Arch Biochem Biophys 303:474–482. https://doi.org/10.1006/abbi.1993.1311
Shirosaki Y, Hirai M, Hayakawa S, Fujii E, Lopes MA, Santos JD, Osaka A (2015) Preparation and in vitro cytocompatibility of chitosan-siloxane hybrid hydrogels. J Biomed Mater Res 103(1):289–299. https://doi.org/10.1002/jbm.a.35171
Zhang M, Hisamori H, Yamada T, Hirano S (1994) 13C CP/MAS NMR spectral analysis of 6-O-tosyl, 6-deoxy-6-iodo, and 6-deoxy derivatives of N-acetylchitosan in a solid state. Biosci Biotech Biochem 58(10):1906–1908. https://doi.org/10.1271/bbb.58.1906
Davis RS, Brough AR, Atkinson A (2003) Formation of silica/epoxy hybrid network polymers. J Non-Cryst Solid 315:197–205. https://doi.org/10.1016/S0022-3093(02)01431-X
Kumamoto K, Maeda T, Hayakawa S, Mustapha NAB, Wang MJ, Shirosaki Y (2021) Antibacterial chitosan nanofiber thin films with bacitracin zinc salt. Polymers 13(7):1–15. https://doi.org/10.3390/polym13071104
Shirosaki Y, Tsuru K, Hayakawa S, Osaka A, Lopes MA, Santos JD, Costa MA, Fernandes MH (2009) Physical, chemical and in vitro biological profile of chitosan hybrid membrane as a function of organosiloxane concentration. Acta Biomater 5(1):346–355. https://doi.org/10.1016/j.actbio.2008.07.022
Zhou J, Xu T, Wang X, Liu C, Liao X, Huang X, Shi B (2017) A low-cost and water resistant biomass adhesive derived from the hydrolysate of leather waste. RSC Adv 7(7):4024–4029. https://doi.org/10.1039/c6ra27132d
Chen S, Hayakawa S, Shirosaki Y, Fujii E, Kawabata K, Tsuru K, Osaka A (2009) Sol-gel synthesis and microstructure analysis of amino-modified hybrid silica nanoparticles from aminopropyltriethoxysilane and tetraethoxysilane. J Am Ceram Soc 92(9):2074–2082. https://doi.org/10.1111/j.1551-2916.2009.03135.x
Liu XM, Maziarz EP, Heiler DJ, Grobe GL (2003) Comparative studies of poly(dimethyl siloxanes) using automated GPC-MALDI-TOF MS and on-line GPC-ESI-TOF MS. J Am Soc Mass Spectrom 14(3):195–202. https://doi.org/10.1016/S1044-0305(02)00908-X
Wang Y, Zhao Y, Sun C, Hu W, Zhao J, Li G, Zhang L, Liu M, Liu Y, Ding F, Yang Y, Gu X (2016) Chitosan degradation products promote nerve regeneration by stimulating Schwann Cell proliferation via miR-27a/FOXO1 axis. Mol Neurobiol 53(1):28–39. https://doi.org/10.1007/s12035-014-8968-2
Obata A, Iwanaga N, Terada A, Jell G, Kasuga T (2017) Osteoblast-like cell responses to silicate ions released from 45S5-type bioactive glass and siloxane-doped vaterite. J Mater Sci 52(15):8942–8956. https://doi.org/10.1007/s10853-017-1057-y
Acknowledgements
We gratefully acknowledge Masumi Kunisue, Center for Instrumental Analysis, Equipment Sharing Sector, Organization for Promotion of Open Innovation, Kyushu Institute of Technology, for TOF-MS measurement. We thank Ashleigh Cooper, PhD, from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Funding
This research was supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI (grant Number JP19H04471).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Kosei Hattori, Satoshi Hayakawa
Rights and permissions
About this article
Cite this article
Hattori, K., Hayakawa, S. & Shirosaki, Y. Effects of the silicon-containing chemical species dissolved from chitosan–siloxane hybrids on nerve cells. J Sol-Gel Sci Technol 104, 606–616 (2022). https://doi.org/10.1007/s10971-022-05814-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-022-05814-z