Abstract
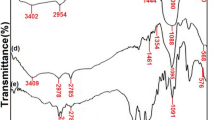
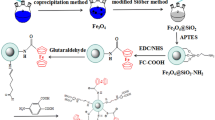
In this work, a novel functionalized magnetic Fe3O4@SiO2 core-shell nanoparticles grafted with carboxymethyl β-cyclodextrin (CM-β-CD) is utilized for adsorption of Pb(II) ions from aqueous solutions. The grafting reaction was performed via carbodimide method by using 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC) and N-hydroxysuccinimide (NHS). The as-prepared nanoadsorbents were characterized by FTIR, SEM, TEM, XRD, TGA, and VSM. The grafting CM-β-CD on the Fe3O4@SiO2 core-shell nanoparticles enhances the ion adsorption capacity due to the strong abilities of the enormous –OH and –COOH functional groups in CM-β-CD to adsorb Pb(II) ions. The effects of factors such as the concentration of β-CD, contact time, initial ion concentration, adsorbent dosage, adsorption kinetics, and isotherms on the adsorption behavior were studied properly. The results depicted that the nanoadsorbents had good adsorption with the maximum adsorption capacity of 170 mg g−1 at 25 °C and pH 6.0. All the equilibrium adsorption kinetics of Pb(II) are fitted well to the pseudo-second-order model. The adsorption data were also found to follow the Langmuir adsorption isotherm model. In summary, the experimental Pb(II) ion adsorption data showed the prepared nanocomposites would be as promising adsorbents to remove various heavy metals from wastewater.

Highlights
-
A magnetic nanocomposite adsorbent based on β-cyclodextrin was synthesized easily.
-
The adsorbent was used for selective removal of Pb(II) ions from contaminated waters.
-
The adsorbent exhibited high adsorption capacity of 170 mg g−1.
-
Adsorption of Pb(II) followed by pseudo-second-order and Langmuir isotherm models.













Similar content being viewed by others
References
Ozay O, Ekici S, Baran Y, Aktas N, Sahiner N (2009) Removal of toxic metal ions with magnetic hydrogels. Water Res 43:4403–4011
Skodras G, Diamantopoulou I, Pantoleontos G, Sakellaropoulos GP (2008) Kinetic studies of elemental mercury adsorption in activated carbon fixed bed reactor. J Hazard Mater 158:1–13
Arora R (2019) Adsorption of heavy metals—A review. Mater Today Proc 18:4745–4750
Wang Q, Gao W, Liu Y, Yuan J, Xu Z, Zeng Q (2014) Simultaneous adsorption of Cu (II) and SO42− ions by a novel silica gel functionalized with a ditopic zwitterionic Schiff base ligand. Chem Eng J 250:55–65
Tang X, Zheng H, Teng H, Sun Y (2014) Chemical coagulation process for the removal of heavy metals from water: a review. Desal Water Treat 57:1–16
Bashir A, Malik LA, Ahad S, Manzoor T, Bhat MA, Dar GN, Pandith AH (2019) Removal of heavy metal ions from aqueous system by ion-exchange and biosorption methods. Environ Chem Lett 17:729–754
Anirudhan TS, Divya L, Ramachandran M (2008) Mercury(II) removal from aqueous solutions and wastewaters using a novel cation exchanger derived from coconut coir pith and its recovery. J Hazard Mater 157:620–627
Khulbe KC, Matsuur T (2018) Removal of heavy metals and pollutants by membrane adsorption techniques. Appl Water Sci 8:19–48
Yoon J, Amy G, Chung J, Sohn J, Yoon Y (2009) Removal of toxic ions (chromate, arsenate, and perchlorate) using reverse osmosis, nanofiltration, and ultrafiltration membranes. Chemosphere 77:228–235
Li Z, Wei Q, Yuan R, Zhou X, Liu H, Shan Q (2007) A new room temperature ionic liquid 1-butyl-3-trimethylsilylimidazolium hexafluorophosphate as a solvent for extraction and preconcentration of mercury with determination by cold vapor atomic absorption spectrometry. Talanta 71:68–72
Khattab IA, Shaffei MF, Shaaban NA, Hussein HS, Abd El-Rehim SS (2013) Electrochemical removal of copper ions from dilute solutions using packed bed electrode. Part ІІ. Egyp J Pet 22:205–210
Andelescu A, Nistor MA, Muntean SG, Rădulescu-Grad ME (2018) Adsorption studies on copper, cadmium, and zinc ion removal from aqueous solution using magnetite/carbon nanocomposites. J Sep Sci Technol 53:2352–2364
Alhumaimess MS (2020) Sulfhydryl functionalized activated carbon for Pb(II) ions removal: kinetics, isotherms, and mechanism. Sep Sci Technol 55:1303–1316
Zhang M, Yin Q, Ji X, Wang F, Gao X, Zhao M (2020) High and fast adsorption of Cd(II) and Pb(II) ions from aqueous solutions by a waste biomass based hydrogel. Sci Rep. 10:3285–3308
El-Kafrawy AF, El-Saeed SM, Farag RK, Al-Aidy El-Saied H, El-Sayed, Abdel-Raouf M (2017) Adsorbents based on natural polymers for removal of some heavy metals from aqueous solution. Egyp J Pet 26:23–32
Mittal V. (2010) Optimization of polymer nanocomposite properties, Wiley-VCH Verlag GmbH & Co., Weinheim, pp. 1–19.
Pavlidou S, Papaspyrides CD (2008) A review on polymer-layered silicate nanocomposites. Prog Polym Sci 33:1119–1198
Sahoo NG, Rana S, Cho JW, Li L, Chan SH (2010) Polymer nanocomposites based on functionalized carbon nanotubes. Prog Polym Sci 35:837–867
Potts JR, Dreyer DR, Bielawski CW, Ruoff RS (2011) Graphene-based polymer nanocomposites. Polymer 52:5–25
Ray SS, Okamoto M (2003) Polymer/layered silicate nanocomposites: a review from preparation to processing. Prog Polym Sci 25:1539–1641
Rebekah A, Bharath G, Naushad M, Viswanathan C, Ponpandian N (2020) Magnetic graphene/chitosan nanocomposite: a promising nano-adsorbent for the removal of 2-naphthol from aqueous solution and their kinetic studies. Int J Biol Macromol 159:530–538
Tan C, Li J, Liu W, Zhao Q, Wang X, Li Y (2020) Core-shell magnetic covalent organic framework nanocomposites as an adsorbent for effervescent reaction-enhanced microextraction of endocrine disruptors in liquid matrices. Chem Eng J 396:125191–125204
Karami S, Zeynizadeh B (2019) Reduction of 4-nitrophenol by a disused adsorbent: EDA-functionalized magnetic cellulose nanocomposite after the removal of Cu2+. Carbohyd Polym 211:298–307
Jiang J, Liu P, Zhao S (2015) Magnetic ATP/FA/Poly(AA-co-AM) ternary nanocomposite microgel as selective adsorbent for removal of heavy metals from wastewater. Colloids Surf A: Physicochem Eng Asp 470:31–38
Pirhaji JZ, Moeinpour F, Mirhoseini Dehabadi A, Yasini, Ardakani SA (2020) Synthesis and characterization of halloysite/graphene quantum dots magnetic nanocomposite as a new adsorbent for Pb(II) removal from water. J Mol Liq 300:112345–112351
Abd Razak NF, Shamsuddin M, Lee SL (2018) Adsorption kinetics and thermodynamics studies of gold(III) ions using thioctic acid functionalized silica coated magnetite nanoparticles. Chem Eng Res Des 130:18–28
He J, Shang H, Zhang X, Sun X (2018) Synthesis and application of ion imprinting polymer coated magnetic multi-walled carbon nanotubes for selective adsorption of nickel ion. Appl Surf Sci 428:110–117
Zhang J, Zhai S, Li S, Xiao Z, Song Y, An Q, Tian G (2013) Pb(II) removal of Fe3O4@SiO2–NH2 core–shell nanomaterials prepared via a controllable sol–gel process. Chem Eng J 215-216:461–471
Candid Balbino TA, Bellato CR, Dias da Silva A, de Oliveira Marques Neto J, de Moura Guimarães L (2020) Magnetic cross-linked chitosan modified with ethylenediamine and β-cyclodextrin for removal of phenolic compounds. Colloids Surf A Physicochem Eng Asp 602:125119–125127
Li D, Chai K, Yao X, Zhou L, Wu K, Huang Z, Yan J, Qin X, Wei W, Ji H (2012) β-Cyclodextrin functionalized SBA-15 via amide linkage as a super adsorbent for rapid removal of methyl blue. J Colloid Inter Sci 583:100–112
Qin X, Bai L, Tan L, Li L, Song F, Wang Y (2019) β-Cyclodextrin-crosslinked polymeric adsorbent for simultaneous removal and stepwise recovery of organic dyes and heavy metal ions: Fabrication, performance and mechanisms. Chem Eng J 372:1007–1018
Liu X (2004) Surface modification and characterization of magnetic polymer nanospheres prepared by miniemulsion polymerization. Langmuir 20:10278–10282
Jiang W, Yang HC, Yang SY, Horng HE, Hung JC, Chen YC, Hong CY (2004) Preparation and properties of superparamagnetic nanoparticles with narrow size distribution and biocompatible. J Magn Magn Mater 283:210–214
Rane AV, Kanny K, Abitha VK, Thomas S (2018) In: Synthesis of inorganic nanomaterials, advances and key technologies, Elsevier Ltd, Chapter 5, PP 121–139.
Yan Q, Zheng HN, Jiang C, Li K, Xiao SJ (2015) EDC/NHS activation mechanism of polymethacrylic acid: anhydride versus NHS-ester. RSC Adv 5:69939–69947
Yang D, Hu J, Fu S (2009) Controlled Synthesis of Magnetite−Silica Nanocomposites via a Seeded Sol-Gel Approach. Phys Chem C 113:7646–7651
Aumaille K, Vallee C, Granier A, Goullet A, Gaboriau F, Turban G (2000) A comparative study of oxygen/organosilicon plasmas and thin SiOxCyHz films deposited in a helicon reactor. Thin Solid Films 359:188–196
Banerjee SS, Chen DH (2008) Cyclodextrin conjugated magnetic colloidal nanoparticles as a nanocarrier for targeted anticancer drug delivery. Nanotechnology 19:265601–265607
Nematidil N, Nezami S, Mirzaie F, Ebrahimi E, Sadeghi M, Farmani N, Sadeghi H (2021) Fabrication and characterization of a novel nanoporous nanoaerogel based on gelatin as a biosorbent for removing heavy metal ions. J Sol-Gel Sci Technol 97:721–733
Fan L, Luo C, Sun M, Qiu H, Li X (2013) Synthesis of magnetic β-cyclodextrin-chitosan/graphene oxide as nanoadsorbent and its application in dye adsorption and removal. Colloid Surf B 103:601–607
Wang D, Liu L, Jiang X, Yu J, Chen X (2015) Adsorption and removal of malachite green from aqueous solution using magnetic β-cyclodextrin-graphene oxide nanocomposites as adsorbents. Colloids Surf A, Physicochem Eng Asp 466:166–173
Zhou Y, Fu S, Zhang L, Zhan H, Levit MV (2014) Use of carboxylated cellulose nanofibrils-filled magnetic chitosan hydrogel beads as adsorbents for Pb(II). Carbohydr Polym 101:75–82
Gupta VK, Attar N, Yola ML, Üstündağ Z, Uzun L (2014) A novel magnetic Fe@Au core–shell nanoparticles anchored graphene oxide recyclable nanocatalyst for the reduction of nitrophenol compounds. Water Res 48:210–217
Sun H, Cao L, Lu L (2011) Magnetite/reduced graphene oxide nanocomposites: one step solvothermal synthesis and use as a novel platform for removal of dye pollutants. Nano Res 4:550–562
Arnal PM, Weidenthaler C, Schüth F (2008) Highly monodisperse zirconia-coated silica spheres and zirconia/silica hollow spheres with remarkable textural properties. Chem Mater 18:2733–2739
Caruntu D, Caruntu G, Chen Y, O’Connor JC, Goloverda G, Kolesnichenko VL (2004) Synthesis of variable-sized nanocrystals of Fe3O4 with high surface reactivity. Chem Mater 25:5527–5534
Salustio PG, Feio G, Figueirinhas JL, Pinto JF, Marques HMC (2009) The influence of the preparation methods on the inclusion of model drugs in a β-cyclodextrin cavity. Eur J Pharm Biopharm 71:377–386
Gunasundari E, Senthil Kumar P (2017) Adsorption isotherm, kinetics and thermodynamic analysis of Cu(II) ions onto the dried algal biomass (Spirulina platensis). J Ind Eng Chem 56:129–144
Anirudhan T, Ramachandran M (2007) Surfactant-modified bentonite as adsorbent for the removal of humic acid from wastewaters. Appl Clay Sci 35:276–281
Cimino G, Passerini A, Toscano G (2000) Removal of toxic cation and Cr(VI) from aqueous solution by hazelnut shell. Water Res 34:2955–2962
Gucek A, Sener S, Bilgen S, Mazmanci ML (2005) Adsorption and kinetic studies of cationic and anionic dyes on pyrophyllite from aqueous solutions. J Colloid Inter Sci 286:53–60
Sanghi R (2002) Bhattacharya B. Review on decolorization of aqueous dye solutions by low cost adsorbents. Color Technol 118:256–269
Yasemin B, Zeki T (2007) Removal of heavy metals from aqueous solution by sawdust adsorption. J Environ Sci 19:160–166
Anwar J, Shafique U, Waheed-uz-Zaman A, Salman M, Dar A, Anwar S (2010) Removal of Pb(II) and Cd(II) from water by adsorption on peels of banana. Bioresour Technol 101:1752–1755
Vilar VJP, Botelho CMS, Boaventura Boaventura PAR (2005) Influence of pH, ionic strength and temperature on lead biosorption by Gelidium and agar extraction algal waste. Process Biochem 40:3267–3275
Largitte L, Laminie J (2005) Modelling the lead concentration decay in the adsorption of lead onto a granular activated carbon. J Environ Chem Eng 3:474–481
Ou HX, Song YJ, Wang Q, Pan JM, Bian WB, Yi CW, Yan YS (2013) Adsorption of lead(II) by silica/cell composites from aqueous solution: kinetic, equilibrium, and thermodynamics studies. Water Environ Res 85:184–191
Tesfaw T, Chekol F, Mehretie S, Admassie S (2016) Adsorption of Pb(II) ions from aqueous solution using lignin from Hagenia abyssinica. Bull Chem Soc Ethiop 30:473–484
Kalak T, Cierpiszewski R (2019) Comparative studies on the adsorption of Pb(II) ions by fl y ash and slag, obtained from CFBC technology. Pol J Chem Technol 21:72–81
Hamadneh I, Abu-Zurayk R, Abu-Irmaileh B, Bozeya A, Al-Dujaili AH (2015) Adsorption of Pb(II) on raw and organically modified Jordanian bentonite. Clay Miner 50:485–496
Cataldo S, Gianguzza A, Milea D, Muratore N, Pettignano A (2016) Pb(II) adsorption by a novel activated carbon alginate composite material. A kinetic and equilibrium study. Int J Biol Macromol 92:769–778
Barsbay M, Akkaş Kavaklı P, Tilki S, Kavaklı C, Güven O (2018) Porous cellulosic adsorbent for the removal of Cd (II), Pb(II) and Cu(II) ions from aqueous media. Radiat Phys Chem 142:70–76
Funding
The authors extend their appreciation to the Payame Noor University for financial support of this work.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by ZJ. The first draft of the manuscript was written by HH and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jahanbakhsh, Z., Hosseinzadeh, H. & Masoumi, B. Synthesis of carboxymethyl β-cyclodextrin bonded Fe3O4@SiO2–NH2 core-shell magnetic nanocomposite adsorbent for effective removal of Pb(II) from wastewater. J Sol-Gel Sci Technol 99, 230–242 (2021). https://doi.org/10.1007/s10971-021-05569-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-021-05569-z