Abstract
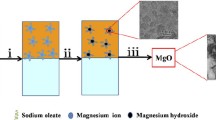
The preparation of MgO nanoparticles with relatively small sizes and highly active surface areas is still a great challenge nowadays. The principal objective of this work was to investigate and optimize the calcined conditions of MgO with smaller sizes and higher surface areas, which contributed to its better degradation ability towards paraoxon pollutants. Firstly, the aerogel Mg(OH)2 nanosheets with an increased surface area of 349.0 m2/g was prepared via a combined sol–gel-hydrothermal method. Then the calcined MgO nanoparticles with different morphology, size dimensions, and specific surface areas were obtained at different calcination temperatures from 400 to 800 °C. The optimized average grain size of MgO obtained at 600 °C was achieved to 8.7 ± 3.2 nm with a relatively high surface area up to 231.4 m2/g. Meanwhile, such as-prepared MgO nanoparticles showed an excellent performance towards paraoxon degradation, and the maximum degradation amount of paraoxon-ethyl was up to 57.8 mg/g@90 min at room temperature. The kinetics of degradation was consistent with the pseudo-second-order model (R2 > 0.99). Such excellent degradation capacity was derived from more generations of superoxide radical (•O2−) sites as revealed by using the nitro blue tetrazolium (NBT) as a probe. The results indicated that the MgO nanoparticles reported herein could be an effective candidate for the environmental remediation of organophosphorus toxin pollutants due to their facile scale-up, low cost, eco-friendly characteristic, and high removal efficiency.








Similar content being viewed by others
Availability of data and material
All data generated or analyzed during this study are included in this published article and its supplementary files.
References
Lim L, Bolstad HM (2019) Organophosphate insecticides: neurodevelopmental effects. Encyclopedia of environmental health 785–791
Katagi T (2010) Bioconcentration, bioaccumulation, and metabolism of pesticides in aquatic organisms. Rev Environ Contam Toxicol 204:1–132
Wang G, Yang Y, Han D, Li Y (2017) Oxygen defective metal oxides for energy conversion and storage. Nano Today 13:23–39
Védrine JC, Importance (2019) Features and uses of metal oxide catalysts in heterogeneous catalysis. Chin J Catal 40(11):1627–1636
Hao J, Ji L, Wu K, Yang N (2018) Electrochemistry of ZnO@reduced graphene oxides. Carbon 130:480–486
He N, Li H, Ji L, Liu X, Zhou H, Chen J (2017) Reusable chromium oxide coating with lubricating behavior from 25 to 1000 °C due to a self-assembled mesh-like surface structure. Surf Coat Technol 321:300–308
Venhryn YI, Savka SS, Bovhyra RV, Zhyrovetsky VM, Serednytski AS, Popovych DI (2021) Obtaining, structure and gas sensor properties of nanopowder metal oxides. Mater Today Proc 35(4):588-594
Fomin A, Fomina M, Koshuro V, Rodionov I (2019) Composite metal oxide coatings on chromium-nickel stainless steel produced by induction heat treatment. Composite Struct 229:111451
Islam MA, Morton DW, Johnson BB (2018) Manganese oxides and their application to metal ion and contaminant removal from wastewater. J Water Process Eng 26:264–280
Babu AT, Antony R (2019) Green synthesis of silver doped nano metal oxides of zinc & copper for antibacterial properties, adsorption, catalytic hydrogenation & photodegradation of aromatics. J Environ Chem Eng 7:102840
Yang Y, Zhang C, Hu Z (2013) Impact of metallic and metal oxide nanoparticles on wastewater treatment and anaerobic digestion. Environ Sci-Process Impacts 15(1):39–48
Madzokerea TC, Karthigeyan A (2017) Heavy metal ion effluent discharge containment using magnesium oxide (MgO) nanoparticles. Mater Today Proc 4(1):9–18
Cai Y, Li C, Wu D, Wang W, Tan F, Wang X, Wong PK, Qiao X (2017) Highly active MgO nanoparticles for simultaneous bacterial inactivation and heavy metal removal from aqueous solution. Chem Eng J 312:158–166
Khursheed A, Shaikh MM (2019) Shape controlled synthesis of high surface area MgO microstructures for highly efficient congo red dye removal and peroxide sensor. J Environ Chem Eng 7(5):103347
Borgohain X, Boruah A, Sarma GK, Rashid MH (2020) Rapid and extremely high adsorption performance of porous MgO nanostructures for fluoride removal from water. J Mol Liq 305:112799
Zhou J, Yang S, Yu J (2011) Facile fabrication of mesoporous MgO microspheres and their enhanced adsorption performance for phosphate from aqueous solutions. Colloids Surf A Physicochem Eng Asp 379(1-3):102–108
Yu XY, Luo T, Jia Y, Zhang Y, Liu J, Huang X (2011) Porous hierarchically micro-/nanostructured MgO: morphology control and their excellent performance in As(III) and As(V) removal. J Phys Chem C 115(45):22242–22250
Chowdhurya AH, Bhanjab P, Salamc N, Bhaumik A, Islam SM (2018) Magnesium oxide as an efficient catalyst for CO2 fixation and N-formylation reactions under ambient conditions. Mol Catal 450:46–54
Kumaril L, Li WZ, Vannoy CH, Leblanc RM, Wang DZ (2009) Synthesis, characterization and optical properties of Mg(OH)2 micro-/nanostructure and its conversion to MgO. Ceram Int 35(8):3355–3364
Gao P, Tian X, Yang C, Zhou Z, Li Y, Wang Y, Komarneni S (2016) Fabrication performance and mechanism of MgO meso-/macroporous nanostructures for simultaneous removal of As(iii) and F in a groundwater system. Environ Sci Nano 3(6):1416–1424
Liu T, Ma PC, Yu JK, Li L, Liu XM (2010) Preparation of MgO by thermal decomposition of Mg(OH)2. J Chin Ceram Soc 38:7
Ganguly A, Trinh P, Ramanujavhary KV, Ahmad T, Mugweru A, Ganguli AK (2011) Reverse micellar based synthesis of ultrafine MgO nanoparticles (8-10 nm): characterization and catalytic properties. J Colloid Interface Sci 353(1):137–142
Alavi MA, Morsali A (2010) Syntheses and characterization of Mg(OH)2 and MgO nanostructures by ultrasonic method. Ultrason Sonochem 17(2):441–446
Cao CY, Qu J, Wei F, Liu H, Song W (2012) Superb adsorption capacity and mechanism of flowerlike magnesium oxide nanostructures for lead and cadmium ions. ACS Appl Mater Interfaces 4(8):4283–4287
Kuang M, Yang G, Xie Z, Su Q, Liu B (2018) Preparation of flower-like MgO via spray drying with high adsorption performance. IOP Conf Ser Mater Sci Eng 423:012085
Karthik K, Dhanuskodi S, Gobinath C, Prabukumarb S, Sivaramakrishnan S (2019) Fabrication of MgO nanostructures and its efficient photocatalytic, antibacterial and anticancer performance. J Photochem Photobiol B: Biol 190:8–20
Possato LG, Pereira E, Gonçalves RGL, Pulcinelli SH, Martins L, Santilli CV (2020) Controlling the porosity and crystallinity of MgO catalysts by addition of surfactant in the sol–gel synthesis. Catal Today 3445:2–58
Tang M, Ren Y, Hu Y, Ye L, Yue B, He H (2018) Synthesis and orientational assemblies of MgO nanosheets with exposed (111) facets. Chin Chem Lett 29(6):935–938
Dhal JP, Sethi M, Mishra BG, Hota G (2015) MgO nanomaterials with different morphologies and their sorption capacity for removal of toxic dyes. Mater Lett 141:267–271
Jia Y, Yu XY, Luo T, Jin Z, Sun B, Liu J, Huang X (2014) Necklace-like mesoporous MgO/TiO2 heterojunction structures with excellent capability for water treatment. Dalton Trans 43(6):2348-–2351
Li X, Xiao W, He G, Zheng W, Yu N, Tan M (2012) Pore size and surface area control of MgO nanostructures using a surfactant-templated hydrothermal process: high adsorption capability to azo dyes. Colloids Surf A: Physicochem Eng Asp 408:79–86
Shandilya M, Rai R, Singh J (2016) Review: hydrothermal technology for smart materials. Adv Appl Ceram 115(6):354–376
Koper OB, Lagadic I, Volodin A, Klabunde KJ (1997) Alkaline-earth oxide nanoparticles obtained by aerogel methods. characterization and rational for unexpectedly high surface chemical reactivities. Chem Mater 9:2468–2480
Klabunde KJ, Stark J, Koper O, Mohs C, Park DG, Decker S, Jiang Y, Lagadic I, Zhang D (1996) Nanocrystals as stoichiometric reagents with unique surface chemistry. J Chem Phys 100:12142–12153
Diao Y, Walawender WP, Sorensen CM, Klabunde KJ, Ricker T (2002) Hydrolysis of magnesium methoxide. effects of toluene on gel structure and gel chemistry. Chem Mater 14:362–368
Pouxviel JC, Boilot JP, Poncelet O, Hubert-Pfalzgraf LG, Lecomte A, Dauger A, Beloeil JC (1987) An aluminosiloxane as a ceramic precursor. J Non-Crystalline Solids 93:277–286
Ueno S, Nakashima K, Sakamoto Y, Wada S (2015) Synthesis of silver-strontium titanate hybrid nanoparticles by sol–gel-hydrothermal method. Nanomaterials 5(2):386–397.
Utamapanya S, Klabunde KJ, Schlup JR (1991) Nanoscale metal oxide particles/clusters as chemical reagents. Synthesis and properties of ultrahigh surface area magnesium hydroxide and magnesium oxide. Chem Mater 3:175–181
Samadi NS, Mustajab MKAA, Yacob AR (2010) Activation temperature effect on the basic strength of prepared aerogel MgO (AP-MgO). Int J Basic Appl Sci 10:54–57
Kim HW, Shim SH (2006) Growth of MgO nanowires assisted by the annealing treatment of Au-coated substrates. Chem Phys Lett 422(1-3):165–169
Jin C, Kim H, Lee WI, Lee C (2011) Ultraintense luminescence in semiconducting- material- sheathed MgO nanorods. Adv Mater 23(17):1982–1987
Cui W, Li P, Wang Z, Zheng S, Zhang Y (2018) Adsorption study of selenium ions from aqueous solutions using MgO nanosheets synthesized by ultrasonic method. J Hazard Mater 341:268–276
Wang LC, Zhang WY, Wang L, Wu HH, Wu D, Lu SY, Huang XP (2021) Synthesis rod-like mesoporous MgO using sodium polystyrenesulfonate (PSS) as structure-direct template and its application to plumbum absorption in waste water. Mater Lett 285:129051
Gajengi AL, Sasaki T, Bhanage BM (2017) Mechanistic aspects of formation of MgO nanoparticles under microwave irradiation and its catalytic application. Adv Powder Technol 28:1185–1192
Moon HR, Urban JJ, Milliron DJ (2009) Size-controlled synthesis and optical properties of monodisperse colloidal magnesium oxide nanocrystals. Angew Chem Int Ed 48(34):6278–6281
Sellik A, Pollet T, Ouvry L, Briançon S, Fessi H, Hartmann DJ, Renaud FNR (2017) Degradation of paraoxon (VX chemical agent simulant) and bacteria by magnesium oxide depends on the crystalline structure of magnesium oxide. Chem-Biol Interact 267:67–73
Wei X, Li C, Wang C, Lin S, Wu J, Guo M (2018) Rapid and destructive adsorption of paraoxon-ethyl toxin via a self-detoxifying hybrid electrospun nanofibrous membrane. Chem Eng J 351:31–39
Xu X, Duan X, Yi Z, Zhou Z, Fan X, Wang Y (2010) Photocatalytic production of superoxide ion in the aqueous suspensions of two kinds of ZnO under simulated solar light. Catal Commun 12(3):169–172
Blelski BHJ, Shiue GG, Bajuk S (1980) Reduction of nitro blue tetrazolium by CO2− and O2− radicals. J Chem Phys 84(8):830–833
Hao YJ, Liu B, Tian LG, Li FT, Ren J, Liu SJ, Liu Y, Zhao J, Wang XJ (2017) Synthesis of {111} facet-exposed MgO with surface oxygen vacancies for ROS generation in the dark. ACS Appl Mater Interfaces 9(14):12687–12693
Giamello E, Murphy D, Garrone E, Zecchina A (1993) Formation of superoxide ions upon oxygen adsorption on magnesium-doped magnesium oxide: an EPR investigation. Spectrochim Acta Part A Mol Biomol Spectrosc 49(9):1323–1330
Acknowledgements
This work was supported by Tianjin Science and Technology Major Project Program (18ZXJMTG00070) for financial supports.
Author information
Authors and Affiliations
Contributions
Shisheng Liu: Data curation, Formal analysis, Investigation, Methodology. Roles/Writing-original draft. Xiaohui Wei: Formal analysis, Investigation. Song Lin: Conceptualization, Funding acquisition, Methodology, Roles/Writing-review & editing, Supervision. Minjie Guo: Roles/Writing-review & editing, Supervision.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Liu, S., Wei, X., Lin, S. et al. Preparation of aerogel Mg(OH)2 nanosheets by a combined sol–gel-hydrothermal process and its calcined MgO towards enhanced degradation of paraoxon pollutants. J Sol-Gel Sci Technol 99, 122–131 (2021). https://doi.org/10.1007/s10971-021-05561-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-021-05561-7