Abstract
Molecular structures of siloxane materials should be highly controlled for achieving advanced functionalities. However, it is still difficult to precisely control the structure of siloxane materials by the sol–gel processing. In the present study, we focused on the silanol groups in the intermediate oligomers and resultant siloxane materials as a key structural unit for controlling the molecular structure. Thermal stability and chemical reactivity of silanol groups were found to be highly dependent on the steric effects of the surrounding side chains and siloxane skeletons. The present work suggests that controlling the steric effects around silanol groups in the intermediate oligomers allows modulating the crosslink density of siloxane skeletons. The selective molecular modification tunes the structure and chemical properties of the resultant siloxane materials.

Highlights
-
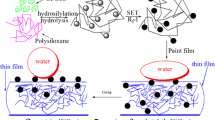
Hydroxyl groups in oraganically modified siloxane oligomers exhibit a diffrent reactivity depending on the local environments.
-
Molecular structures of siloxane materials should be highly controlled for achieving advanced functionalities.
-
The selective molecular modification tunes the structure and chemical properties of the resultant siloxane materials.









Similar content being viewed by others
References
Kim JS, Yang S, Bae BS (2010) Thermally stable transparent sol-gel based siloxane hybrid material with high refractive index for light emitting diode (LED) encapsulation. Chem Mater 22:3549–3555
Mosley DW, Khan arian G, Conner DM, Thorsen DL, Zhang T, Wills M (2013) High refractive index thermally stable phenoxyphenyl and phenylthiophenyl silicones for light-emitting diode applications. J Appl Polym Sci 131(3):39824
Sanchez C, Belleville P, Popall M, Nicole L (2011) Applications of advanced hybrid organic-inorganic nanomaterials: from laboratory to market. Chem Soc Rev 40(2):696–753
O’Shaughnessy WS, Edell DJ, Gleason KK (2009) Initiated chemical vapor deposition of a siloxane coating for insulation of neural probes. Thin Solid Films 517(12):3612–3614
Zhang C, Qu L, Wang Y, Xu T, Zhang C (2018) Thermal insulation and stability of polysiloxane foams containing hydroxyl-terminated polydimethylsiloxanes. RSC Adv 8:9901–9909
Gertz C, Klostermann S, Kochhar SP (2003) Deep frying: the role of water from food being fried and acrylamide formation. OCL 10:297–303
Kabir A, Furton K, Malil A (2013) Innovations in sol-gel microextraction phases for solvent-free sample preparation in analytical chemistry. Trends Analyt Chem 45:197–218
Kitamura N, Fukumi K, Nishi J, Ohno N (2009) Effect of hydroxyl impurity on temperature coefficient of refractive index of synthetic silica glasses. J Non-Crystalline Solid 355:2216–2219
Kuroda M, Tachibana S, Sakamoto N, Okumura S, Nakamura M, Yurimoto H (2018) Water diffusion in silica glass through pathways formed by hydroxyls. Am. Mineral. 103:412–417
Kajihara K, Hirano M, Skuja L, Hosono H (2006) Modification of vacuum-ultraviolet absorption of SiOH groups in SiO2 glass with temperature, F2 laser irradiation, and H-D isotope exchange. J. Non-Crystalline Solid. 352:23–25
Jal P,K, Patel S, Mishra BK (2004) Chemical modification of silica surface by immobilization of functional groups for extractive concentration of metal ions. Talanta. 62:1005–1028
Ju H, Lee D-H, Cho H-C, Kim K-S, Yoon S, Seo S-Y (2014) Application of hydrophilic silanol-based chemical grout for strengthening damaged reinforced concrete flexural members. Materials. 7(6):4823–4844
Valkenberg MH, deCastro C, Holderich WF (2002) Immobilisation of ionic liquids on solid supports. Green Chem 4:88–93
Ide M, El-Roz M, De Canck E, Vicente A, Planckaert T, Bogaerts T, Van Driessche I, Lynen F, Van Speybroeck V, Thybault-Starzyk F, Van Der Voort P (2013) Quantification of silanol sites for the most common mesoporous ordered silicas and organosilicas: total versus accessible silanols. Phys Chem Chem Phys 15(2):642–650
Christy AA, Egeberg, Per K (2005) Quantitative determination of surface silanol groups in silicagel by deuterium exchange combined with infrared spectroscopy and chemometrics. Analyst 130(5):738–744
Chakarova K, Drenchev N, Mihaylov M, Nikolov P, Hadjiivanov K (2013) OH/OD isotopic shift factors of isolated and H-bonded surface silanol groups. J Phys Chem C 117(10):5242–5248
Warring S-L, Beattie D-A, McQuillan A-J (2016) Surficial siloxane-to-silanol interconversion during room-temperature hydration/dehydration of amorphous silica films observed by ATR-IR and TIR-Raman spectroscopy. Langmuir. 32(6):1568–1576
Gallas J-P, Goupil J-M, Vimont A, Lavalley J-C, Gil B, Gilson J-P, Miserque O (2009) Quantification of water and silanol species on various silicas by coupling IR spectroscopy and in-situ thermogravimetry. Langmuir. 25(10):5825–5834
Zhuravlev LT, Potapov VV (2006) Density of silanol groups on the surface of silica precipitated from a hydrothermal solution. Russ J Phys Chem 80(7):1119–1128
Colthup NB, Daly LH, Wiberley SE (1990) In: Introduction to infrared and Raman spectroscopy (3rd ed.), Chapter 12, Academic Press, San Diego, pp 355–385
Harris RK, Robins ML (1978) 29Si nuclear magnetic resonance studies of oligomeric and polymeric siloxanes: 4. Chemical shift effects of end-groups. Polymer 19:1123–1132
Nam K-H, Lee T-H, Bae B-S, Popall M (2006) Condensation reaction of 3-(methacryloxypropyl)-trimethoxysilane and diisobutylsilanediol in non-hydrolytic sol-gel process. J Sol-Gel Sci Technol. 39(3):255–260
Innocenzi P (2003) Infrared spectroscopy of sol–gel derived silica-based films: a spectra-microstructure overview. J Non-Cryst Solids 316:309–319
Malfatti L, Kidchob T, Falcaro P, Costacurta S, Piccinini M, Cestelli Guidi M, Marcelli A, Corrias A, Casula MF, Amenitsch, Innocenzi P (2007) Highly ordered self-assembled mesostructured membranes: porous structure and pore surface coverage. Microporous Mesoporous Mater 103:113–122
Steinbrück N, Pohl S, Kickelbick G (2019) Platinum free thermally curable siloxanes for optoelectronic application—synthesis and properties. RSC Adv 9(4):2205–2216
Jermouni T, Smaihi M, Hovnanian N (1995) Hydrolysis and initial polycondensation of phenyltrimethoxysilane and diphenyldimethoxysilane. J Mater Chem 5(8):1203–1208
van Bommel J, Bernards TNM, Boonstra AH (1991) The influence of the addition of alkyl-substituted ethoxysilane on the hydrolysis—condensation process of TEOS. J Non-Cryst Solids 128:231–242
Bae J-Y, Jang J, Bae B-S (2017) Transparent, thermally stable methyl siloxane hybrid materials using sol-gel synthesized vinyl-methyl oligosiloxane resin. J Sol−Gel Sci Technol 82(1):253–260
Engelhardt G, Jncke H, Lippmaa E, Samoson A (1981) Structure investigations of solid organosilicon polymers by high resolution solid state 29Si NMR. J Organomet Chem 210(3):295–301
Engelhardt G, Jancke H (1981) Structure investigation of organosilicon polymers by silicon-29 NMR. Polym Bull 5:577–584
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Kino, D., Okada, K., Tokudome, Y. et al. Reactivity of silanol group on siloxane oligomers for designing molecular structure and surface wettability. J Sol-Gel Sci Technol 97, 734–742 (2021). https://doi.org/10.1007/s10971-020-05448-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-020-05448-z