Abstract
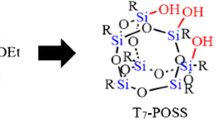
Polyhedral oligomeric silsesquioxanes (POSSs) were prepared by the hydrolytic condensation reaction of organotrialkoxysilanes containing secondary and tertiary amino moieties, as well as quaternary ammonium groups. The employed methodology involved using aqueous trifluoromethanesulfonic acid (HOTf) as the catalyst and solvent at various temperatures and pressures in a Kugelrohr apparatus, which controlled the solvent evaporation time. At lower setting temperature (60 °C) or at shorter solvent evaporation times (≤2 h), the main product was a cage octamer (T8-POSS). Moreover, even at higher temperatures (≥100 °C), the T8 compound was the primary product observed for short evaporation time (≤1.5 h). However, when the solvent evaporation times increased (≥4 h, controlled by pressure) at higher temperatures (≥100 °C), the proportion of a cage decamer (T10-POSS) in the POSS mixtures was larger. Hence, T10-POSS was formed more readily than the corresponding T8 compound when the solvent was evaporated at higher temperatures and over the longer periods of time.

Highlights
-
POSSs containing secondary, tertiary, and quaternary ammonium groups were prepared under various reaction conditions.
-
High proportions of T8-POSS were obtained at lower setting temperatures or shorter solvent evaporation times.
-
Proportion of T10-POSS increased at higher setting temperatures and longer solvent evaporation times.







Similar content being viewed by others
References
Baney RH, Itoh M, Sakakibara A, Suzuki T (1995) Silsesquioxanes. Chem Rev 95:1409–1430
Loy DA, Baugher BM, Baugher CR, Schneider DA, Rahimian K (2000) Substituent effects on the sol-gel chemistry of organotrialkoxysilanes. Chem Mater 12:3624–3632
Laine RM, Roll MF (2011) Polyhedral phenylsilsesquioxanes. Macromolecules 44:1073–1109
Kuo SW, Chang FC (2011) POSS related polymer nanocomposites. Prog Polym Sci 36:1649–1696
Samthong C, Laine RM, Somwangthanaroj A (2013) Synthesis and characterization of organic/inorganic epoxy nanocomposites from poly(aminopropyl/phenyl)silsesquioxanes. J Appl Polym Sci 128:3601–3608
Cordes DB, Lickiss PD, Rataboul F (2010) Recent developments in the chemistry of cubic polyhedral oligosilsesquioxanes. Chem Rev 110:2081–2173
Tanaka K, Chujo Y (2012) Advanced functional materials based on polyhedral oligomeric silsesquioxane (POSS). J Mater Chem 22:1733–1746
Brown JF, Vogt LH, Prescott PI (1964) Preparation and characterization of the lower equilibrated phenylsilsesquioxanes. J Am Chem Soc 86:1120–1125
Clegg W, Sheldrick GM, Vater N (1980) Dodeca(phenylsilasesquioxane). Acta Cryst B36:3162–3164
Roll MF, Kampf JW, Kim Y, Yi E, Laine RM (2010) Nano building blocks via iodination of [PhSiO1.5]n, forming [p-I-C6H4SiO1.5]n (n = 8, 10, 12), and a new route to high-surface-area, thermally stable, microporous materials via thermal elimination of I2 J Am Chem Soc 132:10171–10183
Choi SS, Lee AS, Hwang SS, Baek KY (2015) Structural control of fully condensed polysilsesquioxanes: Ladderlike vs cage structured polyphenylsilsesquioxanes. Macromolecules 48:6063–6070
Ervithayasuporn V, Chimjarn S (2013) Synthesis and isolation of methacrylate- and acrylate-functionalized polyhedral oligomeric silsesquioxanes (T8, T10, and T12) and characterization of the relationship between their chemical structures and physical properties. Inorg Chem 52:13108–13112
Chimjarn S, Kunthom R, Chancharone P, Sodkhomkhum R, Sangtrirutnugul P, Ervithayasuporn V (2015) Synthesis of aromatic functionalized cage-rearranged silsesquioxanes (T8, T10, and T12) via nucleophilic substitution reactions. Dalton Trans 44:916–919
Imoto H, Wada S, Naka K (2016) Efficient isolation of completely decorated polyhedral oligomeric silsesquioxanes by utilizing imine bond formation. Chem Lett 45:1256–1258
Moitra P, Bhagat D, Pratap R, Bhattacharya S (2016) A novel bio-engineering approach to generate an eminent surface-functionalized template for selective detection of female sex pheromone of Helicoverpa armigera. Sci Rep 6:37355
Yu CB, Ren LJ, Wang W (2017) Synthesis and self-assembly of a series of nPOSS-b-PEO block copolymers with varying shape anisotropy. Macromolecules 50:3273–3284
Sharma AK, Sloan R, Ramakrishnan R, Nazarenko SI, Wiggins JS (2018) Structure-property relationships in epoxy hybrid networks based on high mass fraction pendant POSS incorporated at molecular level. Polymer 139:201–212
Naka K, Fujita M, Tanaka K, Chujo Y (2007) Water-soluble anionic POSS-core dendrimer: Synthesis and copper(ii) complexes in aqueous solution. Langmuir 23:9057–9063
Naka K, Sato M, Chujo Y (2008) Stabilized spherical aggregate of palladium nanoparticles prepared by reduction of palladium acetate in octa(3-aminopropyl)octasilsesquioxane as a rigid template. Langmuir 24:2719–2726
Tanaka K, Ishiguro F, Chujo Y (2010) POSS ionic liquid. J Am Chem Soc 132:17649–17651
Ueda K, Tanaka K, Chujo Y (2017) Synthesis of POSS derivatives having dual types of alkyl substituents and their application as a molecular filler for low-refractive and highly durable materials. Bull Chem Soc Jpn 90:205–209
Yuasa S, Sato Y, Imoto H, Naka K (2018) Fabrication of composite films with poly(methyl methacrylate) and incompletely condensed cage-silsesquioxane fillers. J Appl Polym Sci 135:46033
Feher FJ, Wyndham KD (1998) Amine and ester-substituted silsesquioxanes: Synthesis, characterization and use as a core for starburst dendrimers. Chem Commun 323–324
Gravel MC, Zhang C, Dinderman M, Laine RM (1999) Octa(3-chloroammoniumpropyl) octasilsesquioxane. Appl Organomet Chem 13:329–336
Kaneko Y, Shoiriki M, Mizumo T (2012) Preparation of cage-like octa(3-aminopropyl)silsesquioxane trifluoromethanesulfonate in higher yield with a shorter reaction time. J Mater Chem 22:14475–14478
Tokunaga T, Shoiriki M, Mizumo T, Kaneko Y (2014) Preparation of low-crystalline POSS containing two types of alkylammonium groups and its optically transparent film. J Mater Chem C 2:2496–2501
Kaneko Y (2018) Ionic silsesquioxanes: preparation, structure control, characterization, and applications. Polymer 144:205–224
Ishii T, Mizumo T, Kaneko Y (2014) Facile preparation of ionic liquid containing silsesquioxane framework. Bull Chem Soc Jpn 87:155–159
Ishii T, Enoki T, Mizumo T, Ohshita J, Kaneko Y (2015) Preparation of imidazolium-type ionic liquids containing silsesquioxane frameworks and their thermal and ion-conductive properties. RSC Adv 5:15226–15232
Harada A, Koge S, Ohshita J, Kaneko Y (2016) Preparation of a thermally stable room temperature ionic liquid containing cage-like oligosilsesquioxane with two types of side-chain groups. Bull Chem Soc Jpn 89:1129–1135
Maeda D, Ishii T, Kaneko Y (2018) Effect of lengths of substituents in imidazolium groups on the preparation of imidazolium-salt-type ionic liquids containing polyhedral oligomeric silsesquioxane structures. Bull Chem Soc Jpn 91:1112–1119
Liu J, Kaneko Y (2018) Preparation of polyhedral oligomeric silsesquioxanes containing carboxyl side-chain groups and isolation of a cage-like octamer using clay mineral. Bull Chem Soc Jpn 91:1120–1127
Kozuma T, Kaneko Y (2019) Preparation of carboxyl-functionalized polyhedral oligomeric silsesquioxane by a structural transformation reaction from soluble rod-like polysilsesquioxane. J Polym Sci A Polym Chem 57:2511–2518
Hasebe R, Kaneko Y (2019) Control of crystalline-amorphous structures of polyhedral oligomeric silsesquioxanes containing two types of ammonium side-chain groups and their properties as protic ionic liquids. Molecules 24:4553
Janeta M, John Ł, Ejfler J, Szafert S (2015) Novel organic-inorganic hybrids based on T8 and T10 silsesquioxanes: Synthesis, cage-rearrangement and properties. RSC Adv 5:72340–72351
Matsumoto T, Kaneko Y (2018) Selective and high-yielding preparation of ammonium-functionalized cage-like octasilsesquioxanes using superacid catalyst in dimethyl sulfoxide. Chem Lett 47:864–867
Imai K, Kaneko Y (2017) Preparation of ammonium-functionalized polyhedral oligomeric silsesquioxanes with high proportions of cagelike decamer and their facile separation. Inorg Chem 56:4133–4140
Matsumoto T, Kaneko Y (2019) Effect of reaction temperature and time on the preferential preparation of cage octamer and decamer of ammonium-functionalized POSSs. Bull Chem Soc Jpn 92:1060–1067
Park ES, Ro HW, Nguyen CV, Jaffe RL, Yoon DY (2008) Infrared spectroscopy study of microstructures of poly(silsesquioxane)s. Chem Mater 20:1548–1554
Acknowledgements
The authors acknowledge the technical support of Dr. Y. Kusaka (Sekisui Chemical Co., Ltd.) using the Kugelrohr apparatus.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Matsumoto, T., Kaneko, Y. Correlation between molecular structures and reaction conditions (temperature–pressure–time) in the preparation of secondary, tertiary, and quaternary ammonium-functionalized polyhedral oligomeric silsesquioxanes. J Sol-Gel Sci Technol 95, 670–681 (2020). https://doi.org/10.1007/s10971-020-05343-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-020-05343-7