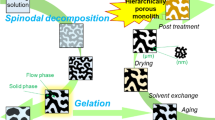
Abstract
A wide-range control over multimodal pore systems in porous monoliths is a key technology for developing functional materials, as the favorable pore structures in different length scales are required to be tailored depending on their application fields. In the alkoxy-derived sol–gel systems of silica and organosilicates, the synthetic methodology of meso- and macroporous monoliths with tunable pore properties has been developed by combining the supramolecular self-assembly of a Pluronic surfactant and polymerization-induced phase-separation techniques. This strategy has been applied to the sol–gel process of phenolic resins, giving rise to the hierarchically porous polymer gels with ordered mesoporosity and the corresponding carbon monoliths after carbonization. However, the controllable size range has been limited so far. This study has explored the relationship between the starting composition and the bimodal pore properties in further detail aiming at a better control of pore properties in phenolic resins. The enlargement of mesopore size has been achieved, yet associated with broadening the mesopore size distribution and coarsening the macropore morphology, resulting in the particle aggregates. The systematical investigation also reveals that the addition of KCl can improve the micelle arrangement in macroframework and provide the narrower mesopore size distribution.

Highlights
-
Meso- and macroporous RF gels are fabricated by the soft-templating and phase separation method.
-
Mechanism for bimodal pore formation in the progression of polycondensation is proposed.
-
Relationship between the starting composition and meso/macropore geometry has been surveyed.
-
The shape of 2D hexagonal building blocks transforms from cylinder to disk by adding KCl.
-
The addition of KCl effectively improves the size distribution of enlarged mesopores.







Similar content being viewed by others
References
Tanaka N, Kobayashi H, Ishizuka N, Minakuchi H, Nakanishi K, Hosoya K, Ikegami T (2002) Monolithic silica columns for high-efficiency chromatographic separations. J Chromatogr A 965:35–49
Guiochon G (2007) Monolithic olumns in high-performance liquid chromatography. J Chromatogr A 1168:101–168
Lozano-Castelló D, Alcañiz-Monge J, de la Casa-Lillo MA, Cazorla-Amorós D, Linares-Solano A (2002) Advances in the study of methane storage in porous carbonaceous materials. Fuel 81:1777–1803
Kokubo T, Kim HM, Kawashita M (2003) Novel bioactive materials with different mechanical properties. Biomaterials 24:2161–2175
Koebel M, Rigacci A, Achard P (2012) Aerogel-based thermal superinsulation: an overview. J Sol–Gel Sci Technol 63:315–339
Hayase G, Kugimiya K, Ogawa M, Kodera Y, Kanamori K, Nakanishi K (2014) The thermal conductivity of polymethylsilsesquioxane aerogels and xerogels with varied pore sizes for practical application as thermal superinsulators. J Mater Chem A 2:6525–6531
Kreutzer MT, Kapteijn F, Moulijn J, Heiszwolf J (2005) Multiphase monolith reactors: chemical reaction engineering of segmented flow in microchannels. Chem Eng J 60:5895–5916
Biener J, Stadermann M, Suss M, Worsley MA, Biener MM, Rose KA, Baumann TF (2011) Advanced carbon aerogels for energy applications. Energy Environ Sci 4:656–667
Minakuchi H, Nakanishi K, Soga N, Ishizuka N, Tanaka N (1996) Octadecylsilylated porous silica rods as separation media for reversed-phase liquid chromatography. Anal Chem 68:3498–3501
Hasegawa G, Kanamori K, Nakanishi K, Abe T (2012) New insights into the relationship between micropore properties, ionic sizes, and electric double-layer capacitance in monolithic carbon electrodes. J Phys Chem C 116:26197–26203
Hasegawa G, Kitada A, Kawasaki S, Kanamori K, Nakanishi K, Kobayashi Y, Kageyama H, Abe T (2015) Impact of electrolyte on pseudocapacitance and stability of porous titanium nitride (TiN) monolithic electrode. J Electrochem Soc 162:A77–A85
Hasegawa G, Deguchi T, Kanamori K, Kobayashi Y, Kageyama H, Abe T, Nakanishi K (2015) High-level doping of nitrogen, phosphorous, and sulfur into activated carbon monoliths and their electrochemical capacitances. Chem Mater 27:4703–4712
Hasegawa G, Kanamori K, Kannari N, Ozaki J, Nakanishi K, Abe T (2015) Hard carbon anodes for Na-ion batteries: toward a practical use. ChemElectroChem 2:1917–1920
Hasegawa G, Kanamori K, Kannari N, Ozaki J, Nakanishi K, Abe T (2016) Studies on electrochemical sodium storage into hard carbons with binder-free monolithic electrodes. J Power Sources 318:41–48
Hasegawa G, Kanamori K, Nakanishi K, Hayashi K (2019) Thermogravimetric evolved gas analysis and microscopic elemental mapping of the solid electrolyte interphase on silicon incorporated in free-standing porous carbon electrodes. Langmuir 35:12680–12688
Hüsing N, Schubert U (1998) Aerogels–airy materials: chemistry, structure, and properties. Angew Chem Int Ed 37:22–45
Stein A, Schroden RC (2001) Colloidal crystal templating of three-dimensionally ordered macroporous solids: materials for photonics and beyond. Curr Opin Solid State Sci 5:553–564
GJAA Soler-Illia, Sanchez C, Lebeau B, Patarin J (2002) Chemical strategies to design textured materials: from microporous and mesoporous oxides to nanonetworks and hierarchical structures. Chem Rev 102:4093–4138
Feinle A, Elsaesser MS, Hüsing N (2016) Sol–gel synthesis of monolithic materials with hierarchical porosity. Chem Soc Rev 45:3377–3399
Yang XY, Chen LH, Li Y, Rooke JC, Sanchez C, Su BL (2017) Hierarchically porous materials: synthesis strategies and structure design. Chem Soc Rev 46:481–558
Kistler SS (1931) Coherent expanded aerogels and jellies. Nature 127:741
Al-Muhtaseb SA, Ritter JA (2003) Preparation and properties of resorcinol–formaldehyde organic and carbon gels. Adv Mater 15:101–114
Pekala RW (1989) Organic aerogels from the polycondensation of resorcinol with formaldehyde. J Mater Sci 24:3221–3227
Liang C, Li Z, Dai S (2008) Mesoporous carbon materials: synthesis and modification. Angew Chem Int Ed 47:3696–3717
Wan Y, Shi Y, Zhao D (2008) Supramolecular aggregates as templates: ordered mesoporous polymers and carbons. Chem Mater 20:932–945
Ma TY, Liu L, Yuan ZY (2013) Direct synthesis of ordered mesoporous carbons. Chem Soc Rev 42:3977–4003
Stein A, Wang Z, Fierke MA (2009) Functionalization of porous carbon materials with designed pore architecture. Adv Mater 21:265–293
Silverstein MS (2014) PolyHIPEs: recent advances in emulsuion-templated porous polymers. Prog Polym Sci 39:199–234
Putz F, Scherer S, Ober M, Morak R, Paris O, Hüsing N (2018) 3D printing of hierarchical porous silica and α-quartz. Adv Mater Technol 3:1800060
Sato Y, Nakanishi K, Hirao K, Jinnai H, Shibayama M, Melnichenko YB, Wignall GD (2001) Colloids Sur A 187–188:117–122
Huesing N, Raab C, Torma V, Roig A, Peterlik H (2003) Periodically mesostructured silica monoliths from diol-modified silanes. Chem Mater 15:2690–2692
Amatani T, Nakanishi K, Hirao K, Kodaira T (2005) Monolithic periodic mesoporous silica with well-defined macropores. Chem Mater 17:2114–2119
Sel O, Kuang D, Thommes M, Smarsly B (2006) Principles of hierarchical meso- and macropore architectures by liquid crystalline and polymer colloid templating. Langmuir 22:2311–2322
Nakanishi K, Kobayashi Y, Amatani T, Hirao K, Kodaira T (2004) Spontaneous formation of hierarchical macro-mesoporous ethane-silica monolith. Chem Mater 16:3652–3658
Nakanishi K, Amatani T, Yano S, Kodaira T (2008) Multiscale templating of siloxane gels via polymerization-induced phase separation. Chem Mater 20:1108–1115
Huang Y, Cai H, Feng D, Gu D, Deng Y, Tu B, Wang H, Webley PA, Zhao D (2008) One-step hydrothermal synthesis of ordered mesostructured carbonaceous monoliths with hierarchical porosities. Chem Commun 2641–2643
Liang C, Dai S (2009) Dual phase separation for synthesis of bimodal meso-/macroporous carbon monoliths. Chem Mater 21:2115–2124
Hao GP, Li WC, Wang S, Wang GH, Qi L, Lu AH (2011) Lysine-assisted rapid synthesis of crack-free hierarchical carbon monoliths with a hexagonal array of mesopores. Carbon 49:3762–3772
Hasegawa G, Kanamori K, Kiyomura T, Kurata H, Abe T, Nakanishi K (2016) Hierarchically porous carbon monoliths comprising ordered mesoporous nanorod assemblies for high-voltage aqueous supercapacitors. Chem Mater 28:3944–3950
Hasegawa G, Shimizu T, Kanamori K, Maeno A, Kaji H, Nakanishi K (2017) Highly flexible hybrid polymer aerogels and xerogels based on resorcinol–formaldehyde with enhanced elastic stiffness and recoverability: insights into the origin of their mechanical properties. Chem Mater 29:2122–2134
Bulavová P, Parmentier J, Slovák V (2018) Facile synthesis of soft-templated carbon monoliths with hierarchical porosity for fast adsorption from liquid media. Micropor Mesopor Mater 272:155–165
Kresge CT, Leonowicz ME, Roth WJ, Vartuli JC, Beck JS (1992) Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature 359:710–712
Huo Q, Margolese DI, Ciesla U, Feng P, Gler TE, Sieger P, Leon R, Petroff PM, Schüth F, Stucky GD (1994) Generalized synthesis of periodic surfactant/inorganic composite materials. Nature 368:317–321
Nakanishi K (1997) Pore structure control of silica gels based on phase separation. J Porous Mater 4:67–112
Nagarajan R (1999) Solubilization of hydrocarbons and resulting aggregate shape transitions in aqueous solutions of Pluronic® (PEO–PPO–PEO) block copolymers. Colloids Surf B 16:55–72
Manet S, Lecchi A, Impéror-Clerc M, Zholobenko V, Durand D, Oliveira CLP, Pedersen JS, Grillo I, Meneau F, Rochas (2011) J Phys Chem B 115:11318–11329
Montalvo G, Valiente M, Rodenas E (1996) Rheological properties of the L phase and the hexagonal, lamellar, and cubic liquid crystals of the CTAB/benzyl alcohol/water system. Langmuir 12:5202–5208
Guo R, Tianqing L, Weili Y (1999) Phase behavior and structure of the sodium dodecyl sulfate/benzyl alcohol/water system. Langmuir 15:624–630
Mitra A, Bhaumik A, Paul BK (2008) Synthesis and characterization of mesoporous titanium dioxide using self-assembly of sodium dodecyl sulfate and benzyl alcohol systems as templates. Micropor Mesopor Mater 109:66–72
Sharp MA, Washington C, Cosgrove T (2010) Solubilisation of model adjuvants by Pluronic block copolymer. J Colloid Interface Sci 344:438–446
Blin JL, Impéror-Clerc M (2013) Mechanism of self-assembly in the synthesis of silica mesoporous materials: in situ studies by X-ray and neutron scattering. Chem Soc Rev 42:4071–4082
Lindén M, Schunk SA, Schüth F (1998) In situ X-ray diffraction study of the initial stages of formation of MCM-41 in a tubular reactor. Angew Chem Int Ed 37:821–823
Frasch J, Leeau B, Soulard M, Patarin J (2000) In situ investigation of cetyltrimethylammonium surfactant/silicate systems, precursors of organized mesoporous MCM-41-type siliceous materials. Langmuir 16:9049–9057
Pevzner S, Regev O, Lind A, Lindén M (2003) Evidence for vesicle formation during the synthesis of catanionic templated mesoscopically ordered silica as studied by cryo-TEM. J Am Chem Soc 125:652–653
Flodström K, Wennerström H, Teixeira C, Amenitsch H, Lindén M, Alfredsson V (2004) Time-resolved in situ studies of the formation of cubic mesoporous silica formed with triblock copolymers. Langmuir 20:10311–10316
Flodström K, Wennerström H, Alfredsson V (2004) Mechanism of mesoporous silica formation. A time-resolved NMR and TEM study of silica-block copolymer aggregation. Langmuir 20:680–688
Flodström K, Teixeira C, Amenitsch H, Alfredsson V, Lindén M (2004) In situ synchrotron small-angle X-ray scattering/X-ray diffraction study of the formation of SBA-15 mesoporous silica. Langmuir 20:4885–4891
Khodakov AY, Zholobenko VL, Impéror-Clerc M, Durand D (2005) Characterization of the initial stages of SBA-15 synthesis by in situ time-resolved small-angle X-ray scattering. J Phys Chem B 109:22780–22790
Manet S, Schmitt J, Impéror-Clerc M, Zholobenko V, Durand D, Oliveira CLP, Pedersen JS, Gervais C, Baccile N, Babonneau F, Grillo I, Meneau F, Rochas C (2011) Kinetics of the formation of 2D-hexagonal silica nanostructured materials by nonionic block copolymer templating in solution. J Phys Chem B 115:11330–11344
Ariga K, Vinu A, Yamauchi Y, Ji Q, Hill JP (2012) Nanoarchitectonics for mesoporous materials. Bull Chem Soc Jpn 85:1–32
Meng Y, Gu D, Zhang F, Shi Y, Cheng L, Feng D, Wu Z, Chen Z, Wan Y, Stein A, Zhao D (2006) A family of highly ordered mesoporous polymer resin and carbon structures from organic-organic self-assembly. Chem Mater 18:4447–4464
Lu AH, Schüth F (2006) Nanocasting: a versatile strategy for creating nanostructured porous materials. Adv Mater 18:1793–1805
Liang C, Li Z, Dai S (2008) Mesoporous carbon materials: synthesis and modification. Angew Chem Int Ed 47:3696–3717
Schuster J, Köhn R, Döblinger M, Keilbach A, Amenitsch H, Bein T (2012) In situ SAXS study on a new mechanism for mesostructure formation of ordered mesoporous carbons: thermally induced self-assembly. J Am Chem Soc 134:11136–11145
Zhang Q, Matsuoka F, Suh HS, Beaucage PA, Xiong S, Smilgies DM, Tan KW, Werner JG, Nealey PF, Wiesner UB (2018) Pathways to mesoporous resin/carbon thin films with alternating gyroid morphology. ACS Nano 12:347–358
Nakanishi K, Soga N (1991) Phase separation in gelling silica-organic polymer solution: systems containing poly (sodium styrenesulfonate). J Am Ceram Soc 74:2518–2530
Nakanishi K, Kanamori K (2005) Organic-inorganic hybrid poly(silsesquioxane) monoliths with controlled macro- and mesopores. J Mater Chem 15:3776–3786
Nakanishi K, Tanaka N (2007) Sol–gel with phase separation. Hierarchically porous materials optimized for high-performance liquid chromatography separations. Acc Chem Res 40:863–873
Hasegawa G, Kanamori K, Nakanishi K, Hanada T (2009) Fabrication of macroporous silicon carbide ceramics by intramolecular carbothermal reduction of phenyl-bridged polysilsesquioxane. J Mater Chem 19:7716–7720
Hasegawa G, Kanamori K, Nakanishi K, Hanada T (2010) A new route to monolithic macroporous SiC/C composites from biphenylene-bridged polysilsesquioxane gels. Chem Mater 22:2541–2547
Kanamori K, Nakanishi K, Hanada T (2006) Rigid macroporous poly(divinylbenzene) monoliths with a well-defined bicontinuous morphology prepared by living radical polymerization. Adv Mater 18:2407–2411
Kanamori K, Hasegawa J, Nakanishi K, Hanada T (2008) Facile synthesis of macroporous cross-linked methacrylate gels by atom transfer radical polymerization. Macromolecules 41:7186–7193
Hasegawa J, Kanamori K, Nakanishi K, Hanada T, Yamago S (2009) Pore formation in poly(divinylbenzene) networks derived from organotellurium-mediated living radical polymerization. Macromolecules 42:1270–1277
Hasegawa J, Kanamori K, Nakanishi K, Hanada T, Yamago S (2009) Rigid crosslinked polyacrylamide monoliths with well-defined macropores synthesized by living polymerization. Macromol Rapid Commun 30:986–990
Hasegawa G, Kanamori K, Nakanishi K, Yamago S (2011) Fabrication of highly crosslinked methacrylate-based polymer monoliths with well-defined macropores via living radical polymerization. Polymer 52:4644–4647
Hasegawa G, Kanamori K, Ishizuka N, Nakanishi K (2012) New monolithic capillary columns with well-defined macropores based on poly(styrene-co-divinylbenzene). ACS Appl Mater Interfaces 4:2343–2347
Smått JH, Schunk S, Lindén M (2003) Versatile double-templating synthesis route to silica monoliths exhibiting a multimodal hierarchical porosity. Chem Mater 15:2354–2361
Weinberger M, Fröschl T, Puchegger S, Peterlik H, Hüsing N (2009) Organosilica monoliths with multiscale porosity: detailed investigation of the influence of the surfactant on structure formation. Silicon 1:19–28
Liang C, Dai S (2006) Synthesis of mesoporous carbon materials via enhanced hydrogen-bonding interaction. J Am Chem Soc 128:5316–5317
Thommes M, Kaneko K, Neimark AV, Olivier JP, Rodriguez-Reinoso F, Rouquerol J, Sing KSW (2015) Pure Appl Chem 87:1051–1069
Ryoo R, Jun S (1997) Improvement of hydrothermal stability of MCM-41 using salt effects during the crystallization process. J Phys Chem B 101:317–320
Zhang W, Glomski B, Pauly TR, Pinnavaia TJ (1999) A new nonionic surfactant pathway to mesoporous molecular sieve silicas with long range framework order. Chem Commun 1803–1804
Zhao D, Yang P, Chmelka BF, Stucky GD (1999) Multiphase assembly of mesoporous-macroporous membranes. Chem Mater 11:1174–1178
Flodström K, Alfredsson V, Källrot N (2003) Formation of a new la-3d cubic meso-structured silica via triblock copolymer-assisted synthesis. J Am Chem Soc 125:4402–4403
Kubo S, Kosuge K (2007) Salt-induced formation of uniform fiberlike SBA-15 mesoporous silica particles and application to toluene adsorption. Langmuir 23:11761–11768
Teixeira CV, Amenitsch H, Linton P, Lindén M, Alfredsson V (2011) The role played by salts in the formation of SBA-15, an in situ small-angle X-ray scattering/diffraction study. Langmuir 27:7121–7131
Booth C, Attwood D (2000) Effects of block architecture and composition on the association properties of poly(oxyalkylene) copolymers in aqueous solution. Macromol Rapid Commun 21:501–527
Leontidis E (2002) Hofmeiseter anion effects on surfactant self-assembly and the formation of mesoporous solids. Curr Opin Colloid Interface Sci 7:81–91
Kabalnov A, Olsson U, Wennerström H (1995) Salt effects on nonionic microemulsions are driven by adsorption/depletion at the surfactant monolayer. J Phys Chem 99:6220–6230
Schmidt-Winkel P, Lukens Jr. WW, Zhao D, Yang P, Chmelka BF, Stucky GD (1999) Mesocellular siliceous foams with uniformly sized cells and windows. J Am Chem Soc 121:254–255
Acknowledgements
This work was supported by a research grant from The Mazda Foundation (No. 17KK-123).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hasegawa, G., Yano, T., Akamatsu, H. et al. Variation of meso- and macroporous morphologies in resorcinol–formaldehyde (RF) gels tailored via a sol–gel process combined with soft-templating and phase separation. J Sol-Gel Sci Technol 95, 801–812 (2020). https://doi.org/10.1007/s10971-020-05236-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-020-05236-9