Abstract
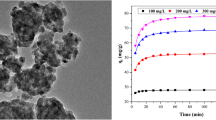
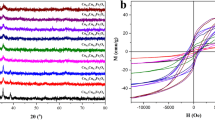
Magnetic MgFe2O4 nanoparticles were prepared successfully via a facile alcohol combustion process and applied to remove the reactive red (RR) from aqueous solution. Characterizations of the as-prepared MgFe2O4 nanoparticles were performed by the scanning electron microscopy (SEM), the transmission electron microscope (TEM), the X-ray diffraction (XRD), the vibrating sample magnetometer (VSM), and the Brunauer–Emmett–Teller (BET) measurement. The adsorption characteristics of RR onto MgFe2O4 nanoparticles at room temperature were investigated. Compared with the pseudo-first-order and intraparticle diffusion model, the pseudo-second-order kinetic model could be applied to evaluate the adsorption performance of RR onto MgFe2O4 nanoparticles in a range of initial concentration of 100–400 mg/L. Based on the values of the correlation coefficients, the equilibrium experimental data related to the adsorption of RR showed that the adsorption performance followed the Langmuir isotherm model with maximum adsorption capacity of 119.07 mg/g. The obtained results implied that the adsorption mechanism of RR onto MgFe2O4 nanoparticles at room temperature was the monolayer adsorption.

Magnetic MgFe2O4 nanoparticles were prepared via the facile alcohol combustion process, and the magnetic MgFe2O4 nanoparticles were applied to remove the reactive red (RR) from aqueous solution, the adsorption kinetics and adsorption isothermals of RR onto MgFe2O4 nanoparticles at room temperature were investigated.
Highlights
-
MgFe2O4 nanoparticles were prepared via the facile alcohol combustion process.
-
The pseudo-second-order kinetics model can explain the adsorption process of RR onto them.
-
The adsorption isotherm of RR onto them at room temperature conformed to Langmuir model.









Similar content being viewed by others
References
Nguyen CH, Juang RS (2019) Efficient removal of methylene blue dye by a hybrid dsorption-photocatalysis process using reduced graphene oxide/titanate nanotube composites for water reuse. J Ind Eng Chem 76:296–309
Pan S, Huang W, Yu QM, Liu X, Liu YH, Liu RJ (2019) A rapid combustion process for the preparation of NixCu1−xFe2O4 nanoparticles and their adsorption characteristics of methyl blue. Appl Phys A Mater. https://doi.org/10.1007/s00339-019-2390-6
Yu Y, Qiao N, Wang DJ, Zhu QZ, Fu F, Cao RQ, Wang R, Liu W, Xu B (2019) Fluffy honeycomb-like activated carbon from popcorn with high surface area and well-developed porosity for ultra-high efficiency adsorption of organic dyes. Bioresource Technol. https://doi.org/10.1016/j.biortech.2019.121340
Chen JX, Jiang MH, Han J, Liu K, Liu ML, Wu, Q (2019) Syntheses of magnetic GO@melamine formaldehyde resin for dyes adsorption. Mater Res Express. https://doi.org/10.1088/2053-1591/ab1ba6
Chen YX, Jing C, Zhang X, Jiang DB, Liu XY, Dong BQ, Feng L, Li SC, Zhang YX (2019) Acid-salt treated CoAl layered double hydroxide nanosheets with enhanced adsorption capacity of methyl orange dye. J Colloid Inter Sci 548:100–109
Du X, Yan H, Dai YJ (2017) Adsorption behavior of neutral red from aqueous solution onto NaOH-modified peanut shell. Fresen Environ Bull 26:4522–4527
Wang JQ, Zhou QS, Song DQ, Qi B, Zhang YJ, Shao YZ, Shao ZQ (2015) Chitosan-silica composite aerogels: preparation, characterization and Congo red adsorption. J Sol-Gel Sci Techn 76(3):501–509
Lorenc-Grabowska E, Gryglewicz G (2007) Adsorption characteristics of Congo Red on coal-based mesoporous activated carbon. Dyes Pigments 74:34–40
Adhikari S, Mandal S, Sarkar D, Kim DH, Madras G (2017) Kinetics and mechanism of dye adsorption on WO3 nanoparticles. Appl Surf Sci 420:472–482
Tian Y, Zhong S, Zhu XJ, Huang AL, Chen YZ, Wang XF (2015) Mesoporous carbon spheres: synthesis, surface modification and neutral red adsorption. Mater Lett 161:656–660
Liu YH, Yu QM, Liu X, Liu RJ (2019) Adsorption characteristics of methyl blue onto magnetic Mn0.5Co0.5Fe2O4 nanoparticles prepared via a rapid combustion process. Environ Prog Sustain 38:S277–S287
Bououdina M, Al-Najar B, Falamarzi L, Vijaya JJ, Shaikh MN, Bellucci S (2019) Effect of annealing on phase formation, microstructure and magnetic properties of MgFe2O4 nanoparticles for hyperthermia. Eur Phys J Plus. https://doi.org/10.1140/epjp/i2019-12485-5
Zhang Y, Zhou ZF, Wen FF, Tan J, Peng T, Lou BQ, Wang HG, Yin SX (2018) A flower-like MoS2-decorated MgFe2O4 nanocomposite: mimicking peroxidase and colorimetric detection of H2O2 and glucose. Sens Actuat B-Chem 275:155–162
Ali NA, Yahya MS, Mustafa NS, Sazelee NA, Idris NH, Ismail M (2019) Modifying the hydrogen storage performances of NaBH4 by catalyzing with MgFe2O4 synthesized via hydrothermal method. Int J Hydrog Energ 44:6720–6727
Wang SF, Li DM, Yang CQ, Sun GG, Zhang J, Xia YH, Xie CM, Yang GX, Zhou M, Liu W (2017) A novel method for the synthesize of nanostructured MgFe2O4 photocatalysts. J Sol-Gel Sci Techn 84(1):169–179
Ivanets A, Roshchina M, Srivastava V, Prozorovich V, Dontsova T, Nahirniak S, Pankov V, Hosseini-Bandegharaei A, Tran HN, Sillanpää M (2019) Effect of metal ions adsorption on the efficiency of methylene blue degradation onto MgFe2O4 as Fenton-like catalysts. Colloid Surf A 571:17–26
Yu QM, Wang Z, Zhang YW, Liu RJ (2019) Covalent immobilization and characterization of penicillin G acylase on amino and GO functionalized magnetic Ni0.5Zn0.5Fe2O4@SiO2 nanocomposite prepared via a novel rapid-combustion process. Int J Biol Macromol 134:507–515
Liu X, Liu RJ, Pan S, Huang W, Fan MM, Li YJ (2019) A facile sol combustion and gel calcination process for the preparation of magnetic MnFe2O4 nanoparticles. J Nanosci Nanotechno 19:5790–5795
Yu QM, Pan S, Huang W, Liu RJ (2019) Effects of solution concentration on magnetic NiFe2O4 nanomaterials prepared via the rapid combustion process. J Nanosci Nanotechno 19:2449–2452
Liu RJ, Yu QM, Liu YH, Liu X, Wang FQ, Xu YN (2018) Optimization of citrate-gel preparation process for magnetic Ni-zn ferrite nanoparticles. J Nanosci Nanotechno 18:2838–2843
Doroftei C, Leontie L (2019) The influence of Sc3+ ions on the microstructure, electrical, and gas-sensing properties of Ni–Co–Sc ferrite. J Sol-Gel Sci Techn 91(3):654–663
Li SS, Liu QF, Lu RZ, Wu XY, Chen J(2018) Effect of solution concentration on magnetic Ni0.5Zn0.5Fe2O4 nanoparticles and their adsorption behavior of neutral red J Nanosci Nanotechnol 18:4798–4804
Khan MI, Akhtar S, Zafar S, Shaheen A, Khan MA, Luque R, Rehman AU (2015) Removal of Congo red from aqueous solution by anion exchange membrane (EBTAC): adsorption kinetics and themodynamics. Materials 8:4147–4161
Yang K, Sun Y (2007) Kinetics of adsorption on carbon from solutions. Biochem Eng J 37:298–310
Sayğılı GA (2015) Synthesis, characterization and adsorption properties of a novel biomagnetic composite for the removal of Congo red from aqueous medium. J Mol Liq 211:515–526
Ding Z, Wang W, Zhang YJ, Li F, Liu JP (2015) Synthesis, characterization and adsorption capability for Congo red of CoFe2O4 ferrite nanoparticles. J Alloy Compd 640:362–370
Jia ZG, Liu JH, Wang QZ, Li SB, Qi Q, Zhu RS (2015) Synthesis of 3D hierarchical porous iron oxides for adsorption of Congo red from dye wastewater. J Alloy Compd 622:587–595
Lafi R, Charradi K, Djebbi MA, Amara ABH, Hafiane A (2016) Adsorption study of Congo red dye from aqueous solution to Mg-Al-layered double hydroxide. Adv Powder Technol 27:232–237
Ji LF, Yu S, Zhou X, Bao YK, Yang FC, Kang WD, Zhang X (2019) Modification of electron structure on the semiconducting single-walled carbon nanotubes for effectively electrosensing guanine and adenine. Anal Chim Acta 1079:86–93
Jawad AH, Mubarak NSA, Abdulhameed AS (2019) Tunable Schiff’s base-cross-linked chitosan composite for the removal of reactive red 120 dye: adsorption and mechanism study. Int J Biol Macromol. https://doi.org/10.1016/j.ijbiomac.2019.10.014
Pereira IC, Carvalho KQ, Passig FH, Ferreira RC, Rizzo-Domingues RCP, Hoppen MI, Perretto F (2018) Thermal and thermal-acid treated sewage sludge for the removal of dye reactive red 120: characteristics, kinetics, isotherms, thermodynamics and response surface methodology design. J Environ Chem Eng 6(6):7233–7246
Prola LD, Acayanka E, Lima EC, Umpierres CS, Vaghetti JC, Santos WO, Djifon PT (2013) Comparison of Jatropha curcas shells in natural form and treated by non-thermal plasma as biosorbents for removal of reactive red 120 textile dye from aqueous solution. Ind Crop Prod 46:328–340
Jawad AH, Mamat NFH, Hameed BH, Ismaila K (2019) Biofilm of cross-linked chitosan-ethylene glycol diglycidyl ether for removal of reactive red 120 and methyl orange: adsorption and mechanism studies. J Environ Chem Eng 7(2):102965
Tabak A, Baltas N, Afsin B, Emirik M, Caglar B, Erend E (2010) Adsorption of reactive red 120 from aqueous solutions by cetylpyridinium-bentonite. J Chem Technol Biot 85:1199–1207
Absalan G, Asadi M, Kamran S, Sheikhian L, Goltz DM (2011) Removal of reactive red-120 and 4-(2-pyridylazo) resorcinol from aqueous samples by Fe3O4 magnetic nanoparticles using ionic liquid as modifier. J Hazard Mater 192:476–484
Pérez-Calderón J, Santosa MV, Zaritzky N (2018) Reactive RED 195 dye removal using chitosan coacervated particles as bio-sorbent: analysis of kinetics, equilibrium and adsorption mechanisms. J Environ Chem Eng 6(5):6749–6760
Dehghani MH, Dehghan A, Najafpoor A (2017) Removing Reactive Red 120 and 196 using chitosan/zeolite composite from aqueous solutionS: kinetics, isotherms, and process optimization. J Ind Eng Chem 51(25):185–195
Acknowledgements
This work was supported by the Opening Project of Key Laboratory of Green Chemistry of Sichuan Institutes of Higher Education (LYJ1910).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yang, Y., Zhang, Y. & Wang, Z. Adsorption characteristics and electrochemical performance of reactive red onto magnetic MgFe2O4 nanoparticles prepared via a facile alcohol combustion process. J Sol-Gel Sci Technol 93, 535–545 (2020). https://doi.org/10.1007/s10971-020-05218-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-020-05218-x