Abstract
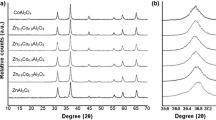
The formation of Zn0.6Co0.4Fe2O4 spinel-based pigments by sol–gel method and post-annealing pathway and the impact of polyol structure on their properties were investigated. The phase evolution and mass changes of gels during annealing were assessed by thermal analysis. The differential thermal analysis revealed that above 400 °C the carboxylate precursors decompose to ZnFe2O4, CoFe2O4, and Zn2SiO4. X-ray diffraction pattern showed the presence of crystalline ZnFe2O4, CoFe2O4, Zn2SiO4, and SiO2, while Fourier transform infrared spectra confirmed the formation of metal-carboxylates and SiO2 matrix at 200 °C and formation of ZnFe2O4, CoFe2O4, and Zn2SiO4 at 1200 °C. The atomic force microscopy images showed spherical nanoparticles of size and shape varying with the polyol structure. The coloring properties and the potential ceramic applications of the obtained dark gray powders were tested by their embedding in opaque and transparent tile glazes and their application on ceramic tile.








Similar content being viewed by others
References
Medeiros PN, Gomes YF, Bomio MRD, Santos IMG, Silva MRS, Paskocimas CA, Li MS, Motta FV (2015) Influence of variables on the synthesis of CoFe2O4 pigment by the complex polymerization method. J Adv Ceram 42:135–141
Cavalcante PMT, Dondi M, Guarini G, Raimondo M, Baldi G (2009) Colour performance of ceramic nano-pigments. Dyes Pigments 80:226–232
Chaudhry AU, Mittal V, Mishra B (2015) Nano nickel ferrite (NiFe2O4) as anti-corrosion pigment for API 5L X-80 steel: An electrochemical study in acidic and saline media. Dyes Pigments 118:18–26
Suppuraj P, Thirunarayanan G, Swaminathan M (2017) Facile synthesis of spinel nanocrystalline ZnFe2O4: enhanced photocatalytic and microbial applications. Mater Sci Appl Chem 34:5–11
El Hadri M, Ahamdane H, El Idrissi Raghni MA (2017) Effect of sol-gel method on colour properties of the classical cobalt olivine (Co2SiO4) ceramic pigment. Bull Mater Sci 40:375–382
Sundararajan M, Kennedy LJ, Aruldoss U, Pasha SK, Vijaya JJ, Dunn S (2015) Microwave combustion synthesis of zinc substituted nanocrystalline spinel cobalt ferrite: Structural and magnetic studies. Mater Sci Semicond Process 40:1–10
Babu BC, Buddhudu S (2014) Analysis of structural and electrical properties of Ni2+:Zn2SiO4 ceramic powders by sol-gel method. J Sol-Gel Sci Technol 70:405–415
Engku Ali EAG, Matori KA, Saion E, Aziz SHA, Zaid MHM, Alibe IM (2018) Structural and optical properties of heat treated Zn2SiO4 composite prepared by impregnation of ZnO on SiO2 amorphous nanoparticles. ASM Sci J 11:75–85
Dippong T, Cadar O, Levei EA, Leostean C, Barbu Tudoran L (2017) Effect of annealing on the structure and magnetic properties of CoFe2O4:SiO2 nanocomposites. Ceram Int 43:9145–9152
Opuchovic O, Kreiza G, Senvaitiene J, Kazlauskas K, Beganskiene A, Kareiva A (2015) Sol-gel synthesis, characterization and application of selected sub-microsized lanthanide (Ce, Pr, Nd, Tb) ferrites. Dyes Pigments 118:176–182
Dippong T, Levei EA, Cadar O, Goga F, Borodi G, Barbu-Tudoran L (2017) Thermal behavior of CoxFe3−xO4/SiO2 nanocomposites obtained by a modified sol-gel method. J Therm Anal Calorim 128:39–52
Ashour AH, El-Batal AI, Maksouda MIA, El-Sayyad GS, Labib S, Abdeltwab E, El-Okr MM (2018) Antimicrobial activity of metal-substituted cobalt ferrite nanoparticles synthesized by sol-gel technique. Particuology 40:141–151
Dippong T, Levei EA, Cadar O, Mesaros A, Borodi G (2017) Sol-gel synthesis of CoFe2O4:SiO2 nanocomposites—insights into the thermal decomposition process of precursors. J Anal Appl Pyrol 125:159–177
Powar RR, Phadtare VD, Parale VG, Park H-H, Pathak S, Kamble PR, Piste PB, Zambare DN (2018) Zambare, Structural, morphological, and magnetic properties of ZnxCo1-xFe2O4 (0≤x≤1) prepared using a chemical co-precipitation method. Ceram Int 44:20782–20789
Jeseentharani V, George M, Jeyaraj B, Dayalan A, Nagaraja KS (2013) Synthesis of metal ferrite (MFe2O4, M=Co, Cu, Mg, Ni, Zn) nanoparticles as humidity sensor materials. J Exp Nanosci 8:358–370
Moeen SJ, Vaezi MR, Yousefi AA (2010) Chemical synthesis of nano-crystalline nickel-zinc ferrite as a magnetic pigment. Prog. Color Color Coat 3:9–17
Dippong T, Levei EA, Cadar O, Goga F, Barbu-Tudoran L, Borodi G (2017) Size and shape-controlled synthesis and characterization of CoFe2O4 nanoparticles embedded in a PVA-SiO2 hybrid matrix. J Anal Appl Pyrol 128:121–130
Joint Committee on Powder Diffraction Standard, International Center for Diffraction Data, 1999.
Bardhan A, Ghosh CK, Mitra MK, Das GC, Mukherjee S, Chattopadhyay KK (2010) Low temperature synthesis of zinc ferrite nanoparticles. Solid State Sci 12:839–844
Rachna A, Singh NB, Agarwal A (2018) Preparation, characterization, properties and applications of nano zinc ferrite. Mater Today: Proc 5:9148–9155
Sutka A, Lagzdina S, Mezinskis G, Pludons A, Vitina I, Timma L (2011) A comparative study of Ni0.7Zn0.3Fe2O4 obtained by sol-gel auto-combustion and flash combustion methods, IOP Confer. Series: Mater. Sci. Eng 25:1–8
Anchieta CG, Cancelier A, Mazutti MA, Jahn SL, Kuhn RC, Gündel A, Chiavone-Filho O, Foletto EL (2014) Effects of solvent diols on the synthesis of ZnFe2O4 particles and their use as heterogeneous photo-Fenton catalysts. Materials 7:6281–6290
Dippong T, Goga F, Levei EA, Cadar O (2019) Influence of zinc substitution with cobalt on thermal behaviour, structure and morphology of zinc ferrite embedded in silica matrix. J Solid State Chem 275:159–166
Acknowledgements
This work was supported by the Ministry of Research and Innovation through Program 1—Development of the National Research and Development System, Subprogram 1.2—Institutional Performance- Projects for Financing Excellence in RDI, Contract No. 19PFE/2018 and a mobility grant of the Romanian Ministry of Research and Innovation, CNCS—UEFISCDI, project number PN-III-P1-1.1-MC-2018-0816, within PNCDI III.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dippong, T., Levei, E.A., Goga, F. et al. The impact of polyol structure on the formation of Zn0.6Co0.4Fe2O4 spinel-based pigments. J Sol-Gel Sci Technol 92, 736–744 (2019). https://doi.org/10.1007/s10971-019-05140-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-019-05140-x