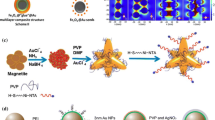
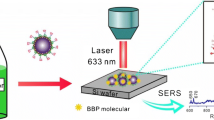
Abstract
With the development of nanotechnology, the preparation method of surface-enhanced Raman scattering (SERS) substrate has made significant progress, which promotes the application of SERS in food safety, environmental protection, and medical testing. We reported on the fabrication of silver-coated magnetic Fe3O4@phenolic resin (Fe3O4@RF) core–shell particles by a facile solvothermal method, followed by deposition of high-density Ag nanoparticles onto the phenolic resin surfaces through an in-situ reduction process. Analysis of Raman spectroscopy was performed by laser Raman spectrometer. The as-synthesized Ag-coated Fe3O4@RF particles as sensitive SERS substrates showed outstanding SERS performances toward probe molecules of 4-aminothiophenolas with a detection limit of 1 × 10–9 mol L–1. Moreover, the enhancement factor of the substrate was calculated to be about 2.86 × 105. Importantly, profiting from the excellent magnetism, the signal attenuated slightly only after three cycles measurements of SERS based on such composite substrates, indicating that Fe3O4@RF@Ag magnetic composite microspheres possessed the outstanding repeatability. On the basis of these results, it is believed that such Fe3O4@RF@Ag substrates are promising for the reusable detection of toxic molecules in practical applications.

Highlights
-
The Fe3O4@RF@Ag composite microspheres were prepared by hydrothermal synthesis method.
-
The composites exhibit SERS performances with a detection limit of 1 × 10–9 mol L–1.
-
The Fe3O4@RF@Ag magnetic microspheres possess the outstanding repeatability.
-
The calculated enhancement factor of the substrate is about 2.86 × 105.








Similar content being viewed by others
References
Fleischmann M, Hendra PJ, McQuillan AJ (1974) Raman spectra of pyridine adsorbed at a silver electrode. Chem Phys Lett 26(2):163–166. https://doi.org/10.1016/0009-2614(74)85388-1
Zhang X, Niu C, Wang Y, Zhou S, Liu J (2014) Gel-limited synthesis of dumbbell-like Fe3O4-Ag composite microspheres and their SERS applications. Nanoscale 6(21):12618–12625. https://doi.org/10.1039/c4nr03301a
Li D, Qu L, Zhai W, Xue J, Fossey JS, Long Y(2011) Facile on-site detection of substituted aromatic pollutants in water using thin layer chromatography combined with surface-enhanced raman spectroscopy.Environ Sci Technol 45(9):4046–4052. https://doi.org/10.1021/es104155r
Qian X, Peng X-H, Ansari DO, Yin-Goen Q, Chen GZ, Shin DM, Yang L, Young AN, Wang MD, Nie S (2008) In vivo tumor targeting and spectroscopic detection with surface-enhanced Raman nanoparticle tags. Nat Biotechnol 26(1):83–90. https://doi.org/10.1038/nbt1377
Kim K, Lee JW, Shin KS (2012) Polyethylenimine-capped Ag nanoparticle film as a platform for detecting charged dye molecules by surface-enhanced raman scattering and metal-enhanced fluorescence. ACS Appl Mater Interfaces 4(10):5498–5504. https://doi.org/10.1021/am3014168
Porter MD, Lipert RJ, Siperko LM, Wang G, Narayanan R (2008) SERS as a bioassay platform: fundamentals, design, and applications. Chem Soc Rev 37(5):1001–1011. https://doi.org/10.1039/b708461g
Camden JP, Dieringer JA, Zhao J, Van Duyne RP (2008) Controlled plasmonic nanostructures for surface-enhanced spectroscopy and sensing. Acc Chem Res 41(12):1653–1661. https://doi.org/10.1021/ar800041s
Ye Y, Liu H, Yang L, Liu J (2012) Sensitive and selective SERS probe for trivalent chromium detection using citrate attached gold nanoparticles. Nanoscale 4(20):6442–6448. https://doi.org/10.1039/c2nr31985c
Yang Y, Liu J, Fu Z-W, Qin D (2014) Galvanic replacement-free deposition of Au on Ag for core-shell nanocubes with enhanced chemical stability and sers activity. J Am Chem Soc 136(23):8153–8156. https://doi.org/10.1021/ja502472x
Bai T, Sun J, Che R, Xu L, Yin C, Guo Z, Gu N (2014) Controllable preparation of core-shell Au-Ag nanoshuttles with improved refractive index sensitivity and SERS activity. ACS Appl Mater Interfaces 6(5):3331–3340. https://doi.org/10.1021/am405357v
Zeng J, Zhao F, Li M, Li C-H, Lee TR, Shih W-C (2015) Morphological control and plasmonic tuning of nanoporous gold disks by surface modifications. J Mater Chem C 3(2):247–252. https://doi.org/10.1039/c4tc02328e
Polte J, Ahner TT, Delissen F, Sokolov S, Emmerling F, Thunemann AF, Kraehnert R (2010) Mechanism of gold nanoparticle formation in the classical citrate synthesis method derived from coupled in situ XANES and SAXS evaluation. J Am Chem Soc 132(4):1296–1301. https://doi.org/10.1021/ja906506j
Braun G, Pavel I, Morrill AR, Seferos DS, Bazan GC, Reich NO, Moskovits M (2007) Chemically patterned microspheres for controlled nanoparticle assembly in the construction of SERS hot spots. J Am Chem Soc 129(25):7760–7761. https://doi.org/10.1021/ja072533e
Guo IW, Pekcevik IC, Wang MCP, Pilapil BK, Gates BD (2014) Colloidal core-shell materials with ‘spiky’ surfaces assembled from gold nanorods. Chem Commun 50(60):8157–8160. https://doi.org/10.1039/c4cc02410a
Li X, Chen G, Yang L, Jin Z, Liu J (2010) Multifunctional Au-coated TiO2 nanotube arrays as recyclable SERS substrates for multifold organic pollutants detection. Adv Funct Mater 20(17):2815–2824. https://doi.org/10.1002/adfm.201000792
Bersani D, Lottici PP, Lopez T, Ding X-Z (1998) A Raman scattering study of PbTiO3 and TiO2 obtained by sol-gel. J Sol-Gel Sci Technol 13(1/2/3):849–853
Liu B, Bai C, Zhao D, Liu W-L, Ren M-M, Liu Q-Z, Yang Z-Z, Wang X-Q, Duan X-L (2016) Novel ferroferric oxide/polystyrene/silver core-shell magnetic nanocomposite microspheres as regenerable substrates for surface-enhanced Raman scattering. Appl Surf Sci 364:628–635. https://doi.org/10.1016/j.apsusc.2015.12.186
Bourgeat-Lami E, Lansalot M (2010) Organic/inorganic composite latexes: the marriage of emulsion polymerization and inorganic chemistry. Adv Polym Sci 233:53–123. https://doi.org/10.1007/12_2010_60
Kong L, Lu X, Bian X, Zhang W, Wang C (2011) Constructing carbon-coated Fe3O4 microspheres as antiacid and magnetic support for palladium nanoparticles for catalytic applications. ACS Appl Mater Interfaces 3(1):35–42. https://doi.org/10.1021/am101077a
Ma W, Xu S, Li J, Guo J, Lin Y, Wang C (2011) Hydrophilic dual-responsive magnetite/PMAA core/shell microspheres with high magnetic susceptibility and ph sensitivity via distillation-precipitation polymerization. J Polym Sci, Part A 49(12):2725–2733. https://doi.org/10.1002/pola.24705
Salazar S, Guerra D, Yutronic N, Jara P (2018) Removal of aromatic chlorinated pesticides from aqueous solution using β-cyclodextrin polymers decorated with Fe3O4 nanoparticles. Polym (Basel, Switz) 10(9):1038/1031–1038/1019. https://doi.org/10.3390/polym10091038
Wang T, Hu X, Dong S (2008) A renewable SERS substrate prepared by cyclic depositing and stripping of silver shells on gold nanoparticle microtubes. Small 4(6):781–786. https://doi.org/10.1002/smll.200700888
He X, Wang H, Zhang Q, Li Z, Wang X (2014) Exotic 3D hierarchical ZnO-Ag hybrids as recyclable surface-enhanced Raman scattering substrates for multifold organic pollutant detection. Eur J Inorg Chem 2014(14):2432–2439. https://doi.org/10.1002/ejic.201402014
Wen G, Zhong B, Huang X, Yu H, Zhang X, Zhang T, Bai H (2010) Novel BN hollow microspheres with open mouths - Facile synthesis, growth mechanism, resonant Raman scattering effect, and cathodoluminescence performance. Eur J Inorg Chem 35:5538–5544. https://doi.org/10.1002/ejic.201000703
Ren J, Tilley RD (2007) Shape-controlled growth of platinum nanoparticles. Small 3(9):1508–1512. https://doi.org/10.1002/smll.200700135
Gu H, Yang Z, Gao J, Chang CK, Xu B (2005) Heterodimers of nanoparticles: formation at a liquid-liquid interface and particle-specific surface modification by functional molecules. J Am Chem Soc 127(1):34–35. https://doi.org/10.1021/ja045220h
Polte J, Tuaev X, Wuithschick M, Fischer A, Thuenemann AF, Rademann K, Kraehnert R, Emmerling F (2012) Formation mechanism of colloidal silver nanoparticles: analogies and differences to the growth of gold nanoparticles. ACS Nano 6(7):5791–5802. https://doi.org/10.1021/nn301724z
Liu X, Atwater M, Wang J, Dai Q, Zou J, Brennan JP, Huo Q (2007) A study on gold nanoparticle synthesis using oleylamine as both reducing agent and protecting ligand. J Nanosci Nanotechnol 7(9):3126–3133. https://doi.org/10.1166/jnn.2007.805
Ge T, Tang K, Yu Y, Tan X (2018) Preparation and properties of the 3-pentadecyl-phenol in situ modified foamable phenolic resin. Polym 10(10):1124/1121–1124/1116. https://doi.org/10.3390/polym10101124
Streckova M, Sopcak T, Medvecky L, Bures R, Faberova M, Batko I, Briancin J (2012) Preparation, chemical and mechanical properties of microcomposite materials based on Fe powder and phenol-formaldehyde resin. Chem Eng J (Amst, Neth) 180:343–353. https://doi.org/10.1016/j.cej.2011.11.036
Beda A, Taberna P-L, Simon P, Matei Ghimbeu C (2018) Hard carbons derived from green phenolic resins for Na-ion batteries. Carbon 139:248–257. https://doi.org/10.1016/j.carbon.2018.06.036
Wang X, Huang H, Li G, Liu Y, Huang J, Yang D-P (2014) Hydrothermal synthesis of 3D hollow porous Fe3O4 microspheres towards catalytic removal of organic pollutants. Nanoscale Res Lett 9(1):1–5. https://doi.org/10.1186/1556-276x-9-648
Baharin SNA, Sarih NM, Mohamad S (2016) Novel functionalized polythiophene-coated Fe3O4 nanoparticles for magnetic solid-phase extraction of phthalates. Polym 8(5):117/111–117/118. https://doi.org/10.3390/polym8050117
Liu J, Sun Z, Deng Y, Zou Y, Li C, Guo X, Xiong L, Gao Y, Li F, Zhao D (2009) Highly water-dispersible biocompatible magnetite particles with low cytotoxicity stabilized by citrate groups. Angew Chem, Int Ed 48(32):5875–5879. https://doi.org/10.1002/anie.200901566
You H, Ji Y, Wang L, Yang S, Yang Z, Fang J, Song X, Ding B (2012) Interface synthesis of gold mesocrystals with highly roughened surfaces for surface-enhanced Raman spectroscopy. J Mater Chem 22(5):1998–2006. https://doi.org/10.1039/c1jm13211c
Kang JS, Hwang SY, Lee CJ, Lee MS (2002) SERS of dithiocarbamate pesticides adsorbed on silver surface; thiram. Bull Korean Chem Soc 23(11):1604–1610. https://doi.org/10.5012/bkcs.2002.23.11.1604
Sanchez-Cortes S, Vasina M, Francioso O, Garcia-Ramos JV (1998) Raman and surface-enhanced Raman spectroscopy of dithiocarbamate fungicides. Vib Spectrosc 17(2):133–144. https://doi.org/10.1016/s0924-2031(98)00025-3
Le RuEC, Blackie E, Meyer M, Etchegoin PG (2007) Surface enhanced Raman scattering enhancement factors: a comprehensive study. J Phys Chem C 111(37):13794–13803. https://doi.org/10.1021/jp0687908
Hildebrandt P, Stockburger M (1984) Surface-enhanced resonance Raman spectroscopy of Rhodamine 6G adsorbed on colloidal silver. J Phys Chem 88(24):5935–5944. https://doi.org/10.1021/j150668a038
Su K-H, Durant S, Steele JM, Xiong Y, Sun C, Zhang X (2006) Raman enhancement factor of a single tunable nanoplasmonic resonator. J Phys Chem B 110(9):3964–3968. https://doi.org/10.1021/jp055566u
Minh N, Phuong DTT (2010) Structural and low temperature Raman scattering studies in SrTi1-xCoxO3 nanoparticles synthesized by sol-gel method. J Sol-Gel Sci Technol 56(3):264–269. https://doi.org/10.1007/s10971-010-2302-x
Chiang C-Y, Liu T-Y, Su Y-A, Wu C-H, Cheng Y-W, Cheng H-W, Jeng R-J (2017) Au nanoparticles immobilized on honeycomb-like polymeric films for surface-enhanced raman scattering (SERS) detection. Polym 9(3):93/1–93/17. https://doi.org/10.3390/polym9030093
Acknowledgements
The work described in this paper was supported by Shandong Province Natural Science Foundation (ZR2012EMM009 and ZR2018MEM012), the Foundation of Key Laboratory of Pulp and Paper Science and Technology of Ministry of Education/Shandong Province of China (No. KF201602), the Scientific Research Foundation for the Returned Overseas Scholars in Jinan (20100406), National Training Program of Innovation and Entrepreneurship for Undergraduates (201610431033 and 201810431008), Qilu University of Technology International Cooperation Fund (QLUTGJHZ2018025), and National Natural Science Foundations of China (31570566, 31500489, 51403111, and 51503107).
Author contributions
W-LL and F-GK supervised this research; including originality, experimental design, data analysis, and manuscript writing; Y-FW, M-MR, and S-JW assisted D-SL in all related areas. KY checked some experiments.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, DS., Liu, B., Wang, YF. et al. Magnetic ferroferric oxide/phenolic resin/silver core–shell nanocomposite as recyclable substrates for enhancing surface-enhanced Raman scattering. J Sol-Gel Sci Technol 92, 124–133 (2019). https://doi.org/10.1007/s10971-019-05093-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-019-05093-1