Abstract
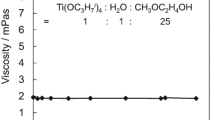
Titanium tetraisopropoxide was hydrolyzed in tetrahydrofuran (THF), 1-butanol, or 2-butanol to prepare coating solutions for titania thin films. The compositions of the starting solutions were so simple, Ti(OC3H7i)4:H2O:solvent = 1:(0.5 or 1.0):25 in mole ratios, that they contained no chelating or peptizing agents. In spite of the absence of chelating or peptizing agents, transparent sols were obtained except for the THF solution of H2O/Ti(OC3H7i)4 = 1.0. All the transparent sols showed high stability in viscosity at least over 1 month. Titania thin films were prepared from the sols by spin- or dip-coating on Si(100) substrates via heat treatment at 700 or 900 °C. The stability of such additive-free sols was examined by comparing the thickness, refractive index, crystalline phase, crystallite size, and microstructure between films that were prepared from sols aged for 1, 5, and 30 days at room temperature. The thickness, refractive index, and crystallite size did not change noticeably with sol aging time. The films prepared from the THF-containing sols were anatase irrespective of the sol-aging time and heat treatment temperature. On the other hand, rutile phase was also observed when the films were prepared from 30-day-aged, 1-butanol-, or 2-butanol-containing sols, followed by firing at 900 °C. The films from the 2-butanol-containing sols also exhibited a change in microstructure at such a long sol aging time. These suggest that the 1-butanol- or 2-butanol-containing sols are unstable during aging while the THF-containing sol is stable as coating solution.

Highlights
-
Simple “titanium alkoxide–water–solvent” solutions were presented as titania coating solutions.
-
The solutions contain neither peptizing nor chelating agents.
-
The solvent was either tetrahydrofuran (THF), 1-butanol or 2-butanol.
-
All the solutions exhibited stability in viscosity.
-
Pot life was the longest for the THF-containing sol in terms of anatase film formation.







Similar content being viewed by others
References
Farrokhpay S, Morris GE, Fornasiero D, Self P (2006) Titania pigment particles dispersion in water-based paint films. J Coat Techn Res 3:275–283
Jeong SH, Kim JK, Kim BS, Shim SH, Lee BT (2004) Characterization of SiO2 and TiO2 films prepared using RF magnetron sputtering and their application to anti-reflective coating. Vacuum 76:507–515
Kako T, Umezama N, Xie K, Ye J (2013) Undoped visible-light-sensitive titania photocatalyst. J Mater Sci 48:108–114
Negishi N, Takeuchi K (2001) Preparation of TiO2 thin film photocatalysts by dip-coating using a highly viscous solvent. J Sol-Gel Sci Technol 22:23–31
Saini KK, Sharma SD, Chanderkant, Kar M, Singh D, Sharma CP (2007) Structural and optical properties of TiO2 thin films derived by sol-gel dip coating process. J Non-Cryst Solids 353:2469–2473
Pettit RB, Brinker CJ, Ashley CS (1985) Sol-gel double-layer antireflection coatings for silicon solar cells. Sol Cells 15:267–278
Yoko T, Kamiya K, Yuasa A, Sakka S (1986) Formation of H2O2 at the illuminated TiO2 film electrode prepared by the sol-gel method and its chemical states. J Electroanal Chem 209:399–404
Doeuff S, Henry M, Sanchez C, Livage J (1987) Hydrolysis of titanium alkoxides: modification of the molecular precursor by acetic acid. J Non-Cryst Solids 89:206–216
Yamazaki S, Uchiyama H, Kozuka H (2018) Additive-free alkoxide–water–alcohol solutions as precursors for crystalline titania thin films. J Sol-Gel Sci Technnol 87:537–543
Ohya Y, Saiki H, Tanaka T, Takahashi Y (1996) Microstructure of TiO2 and ZnO films fabricated by the sol-gel method. J Am Ceram Soc 79:825–830
Committee of Fain Seramikkusu Jiten (ed) Fain Seramikkusu Jiten (Fine Ceramics Dictionary), Gihodo Shuppan, Tokyo, 1987, p 317
Artaki I, Zerda TW, Jonas J (1986) Solvent effects on the condensation stages of the sol-gel process. J Non-Cryst Solids 81:381–395
Acknowledgements
TP thanks the Center of Excellence on Petrochemical and Materials Technology, Chulalongkorn University for the scholarship and financial supports.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Piwluang, T., Hayashido, T., Koizumi, Y. et al. Simple “titanium alkoxide–water–solvent” solutions as titania coating solutions containing no peptizing or chelating agents. J Sol-Gel Sci Technol 92, 57–65 (2019). https://doi.org/10.1007/s10971-019-05088-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-019-05088-y