Abstract
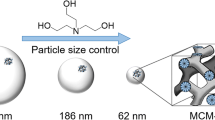
In this paper, we report the fundamental method for controlling monodispersed silica nanoparticles size ranged from 10 to ~135 nm assisted by lysine or lysine hydrochloride, during which the seed regrowth technique was utilized. The synthesis was prepared in the water-base system, which was environment friendly and convenient. According to our research, the influence factors of co-solvent system, molar content of additional TEOS, and molar ratio composition of additional TEOS/seeds were confirmed. The final morphology and size of silica nanoparticles can be tuned by changing the amount of additional TEOS and the seeds size. To guarantee the monodispersity of final particles. In the regrowth process, cyclohexane used as the co-solvent floated on the surface of water to block additional TEOS contact with water directly, and made the silicate intermedium consumable for the growth of the parent particles immediately, while not forming new nucleation. In such a condition, to lower the cost and prepare mass production, the lysine was replaced by lysine hydrochloride, meanwhile, triethylamine was introduced to neutralize the hydrochloride. It should be noted that as the particle size increased to be more than 100 nm, when usinglysine hydrochloride and triethylamine, the types of nitrogen adsorption–desorption isotherms changed from H1 to H3; in contrast, the isotherms of the particles were changed from H2 to H1 when catalyzed by lysine.

Highlights
-
The size of monodisperse silica nanoparticles could be controlled in the range from 10 to 110 nm, assisted by lysine. As the particle size increased, the type of channel changed from “bottleneck hole” to “cylindrical hole”.
-
The size of monodisperse silica nanoparticles could be controlled in the range from 55 to 135 nm, assisted by lysine hydrochloride and triethylamine. As the particle size increased, the channel type changed from “cylindrical hole” to “slit hole”.
-
The optimum molar ratio composition of TEOS/seeds was 80.
-
The optimum molar content of additional TEOS was 2.










Similar content being viewed by others
References
Gao T, Jelle BP, Sandberg LI, Gustavsen A (2013) Monodisperse hollow silica nanospheres for nano insulation materials: synthesis, characterization, and life cycle assessment. ACS Appl Mater Interfaces 5(3):761–767
Neaime C, Amela-Cortes M, Grasset F, Molard Y, Cordier S, Dierre B, Mortier M, Takei T, Takahashi K, Haneda H, Verelst M, Lechevallier S (2016) Time-gated luminescence bioimaging with new luminescent nanocolloids based on [Mo6I8(C2F5COO)6]2- metal atom clusters. Phys Chem Chem Phys 18(43):30166–30173
Eisuke Y, Masaki K, Takuya T, Kazuyuki K (2014) Preparation of size-controlled monodisperse colloidal mesoporous silica nanoparticles and fabrication of colloidal crystals. Chem Mater 26(9):2927–2933
Xia Y, Nguyen TD, Yang M, Lee B, Santos A, Podsiadlo P, Tang Z, Glotzer SC, A KN (2011) Self-assembly of self-limiting monodisperse supraparticles from polydisperse nanoparticles. Nat Nanotechnol 6:580–587
Zoppellaro G, Kolokithas-Ntoukas A, Polakova K, Tucek J, Zboril R, Loudos G, Fragogeorgi E, Diwoky C, Tomankova K, Avgoustakis K, Kouzoudis D, Bakandritsos A (2014) Theranostics of epitaxially condensed colloidal nanocrystal clusters, through a soft biomineralization route. Chem Mater 26(6):2062–2074
Watanabe R, Yokoi T, Kobayashi E, Otsuka Y, Shimojima A, Okubo T, Tatsumi T (2011) Extension of size of monodisperse silica nanospheres and their well-ordered assembly. J Colloid Interface Sci 360(1):1–7
Korzeniowska B, Nooney R, Wencel D, McDonagh C (2013) Silica nanoparticles for cell imaging and intracellular sensing. Nanotechnology 24(44):442002
Liu B, Zeng F, Wu G, Wu S (2011) A FRET-based ratiometric sensor for mercury ions in water with multi-layered silica nanoparticles as the scaffold. Chem Commun 47(31):8913–8915
Cao H, Hu X, Hu C, Zhang Y, Jia N (2013) A novel solid-state electrochemiluminescence sensor for melamine with Ru(bpy)3 2+/mesoporous silica nanospheres/Nafion composite modified electrode. Biosens Bioelectron 41:911–915
Lei J, Wang L, Zhang J (2010) Ratiometric pH sensor based on mesoporous silica nanoparticles and Forster resonance energy transfer. Chem Commun 46(44):8445–8447
Dong Z, Le X, Li X, Zhang W, Dong C, Ma J (2014) Silver nanoparticles immobilized on fibrous nano-silica as highly efficient and recyclable heterogeneous catalyst for reduction of 4-nitrophenol and 2-nitroaniline. Appl Catal B 158-159:129–135
Zhang S, Chen L, Zhou S, Zhao D, Wu L (2010) Facile synthesis of hierarchically ordered porous carbon viain situself-assembly of colloidal polymer and silica spheres and its use as a catalyst support. Chem Mater 22(11):3433–3440
Wang Y, Zhao Q, Han N, Bai L, Li J, Liu J, Che E, Hu L, Zhang Q, Jiang T, Wang S (2015) Mesoporous silica nanoparticles in drug delivery and biomedical applications. Nanomed Nanotechnol Biol Med 11(2):313–327
Prevo BG, Hwang Y, Velev OD (2005) Convective assembly of antireflective silica coatings with controlled thickness and refractive index. Chem Mater 17:3642–3551
Setyowatia E, Amalia SF, Nazriati, Affandi S, Yuwanae M, Setyawan H (2014) Hydrophobic silica coating based on waterglass on copper by electrophoretic deposition. Appl Mech Mater 493:749–754
Kuai S, Badilescu S, Bader G, Briining R, Hu X, Vo-Van T (2003) Preparation of large-area 3D ordered marcroporous titania films by silica colloidal crystal templating. Adv Mater 15:73–75
Li Z, Zhang Y (2006) Monodisperse silica-coated polyvinylpyrrolidone/NaYF4 nanocrystals with multicolor upconversion fluorescence emission. Angew Chem 45(46):7732–7735
Ohno K, Morinaga T, Koh K, Tsujii Y, Fukuda T (2005) Synthesis of monodisperse silica particles coated with well-defined, high-density polymer brushes by surface-initiated atom transfer radical polymerization. Macromolecules 38:2137–2142
Manchanda CK, Khaiwal R, Mor S (2017) Application of sol–gel technique for preparation of nanosilica from coal powered thermal power plant fly ash. J Sol-Gel Sci Technol 83(3):574–581
Rahman IA, Padavettan V (2012) Synthesis of silica nanoparticles by sol-gel: size-dependent properties, surface modification, and applications in silica-polymer nanocomposites—a review. J Nanomater 2012:1–15
Stöber W, FINK A (1968) Controlled growth of monodisperse silica spheres in the micron size range. J Colloid Interface Sci 26:62–69
Tadanaga K, Morita K, Mori K, Tatsumisago M (2013) Synthesis of monodispersed silica nanoparticles with high concentration by the Stöber process. J Sol-Gel Sci Technol 68(2):341–345
Cha JN, Stucky GD, Deming TJ (2000) Biomimetic synthesis of ordered silica structures mediated by block copolypeptides. Nature 403:289–292
Davis TM, Snyder MA, Krohn JE, Tsapatsis M (2006) Nanoparticles in lysine-silica sols. Chem Mater 18:5814–5816
Fan W, Snyder MA, Kumar S, Lee PS, Yoo WC, McCormick AV, Lee Penn R, Stein A, Tsapatsis M (2008) Hierarchical nanofabrication of microporous crystals with ordered mesoporosity. Nat Mater 7(12):984–991
Kung SC, Chang CC, Fan W, Snyder MA (2014) Template-free ordered mesoporous silicas by binary nanoparticle assembly. Langmuir 30(39):11802–11811
Yokoi T, Sakamoto Y, Terasaki O, Kubota Y, Okubo T, Tatsumi T (2006) Periodic arrangement of silica nanospheres assisted by amino acids. J Am Chem Soc 128:13664–13665
Yokoi T, Wakabayashi J, Otsuka Y, Fan W, Iwama M, Watanabe R, Aramaki K, Shimojima A, Tatsumi T, Okubo T (2009) Mechanism of formation of uniform-sized silica nanospheres catalyzed by basic amino acids. Chem Mater 21(15):3719–3729
Brinker CJ (1988) Hydrolysis and condensation of silicates: effects on structure. J Non-Cryst Solids 100:31–50
Giesche H (1994) Synthesis of monodispersed silica powders II. Controlled growth reaction and continuous production process. J Eur Ceram Soc 14:205–214
Wang J, Sugawara-Narutaki A, Fukao M, Yokoi T, Shimojima A, Okubo T (2011) Two-phase synthesis of monodisperse silica nanospheres with amines or ammonia catalyst and their controlled self-assembly. ACS Appl Mater interfaces 3(5):1538–1544
Hartlen KD, Athanasopoulos APT, Kitaev V (2008) Facile preparation of highly monodisperse small silica spheres (15 to >200 nm) suitable for colloidal templating and formation of ordered arrays. Langmuir 24:1714–1720
Hu B, Zeng Z, Hong X (2016) Pore size controlled synthesis of SiO2 colloidal crystal. J Porous Mater 23(3):845–850
Bernards TNM, Van Bommel MJ, Boonstra AH (1991) Hydrolysis-condensation processes of the tetra-alkoxysilanes TPOS, TEOS and TMOS in some alcoholic solvents J Non-Cryst Solids 134:1–13
Sing KSW, Everett DH, Haul RAW, Moscou L, Pierotti RA, Rouquerol J, Siemieniewska T (1985) Reporting physisorption data for gassolid systems with special reference to the determination of surface area and porosity. Pure Appl Chem 57(4):603–619
Matsoukas T, Gulari E (1991) Self-sharpening distributions revisited-polydispersity in growth by monomer addition. J Colloid Interface Sci 145:557–562
Acknowledgements
This work was supported by the National Natural Science Foundation of China (NNSFC Grants 51803104, 51603113) and the Natural Science Foundation of Shandong Province (ZR2016EMP03, ZR2016XJ004). We also thank China Postdoctoral Fund (2019M652154) and the Opening Project of State Key Laboratory of Polymer Materials Engineering (Sichuan University) (sklpme2019-4-28) for their support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
About this article
Cite this article
Xia, L., Yao, B., Shi, H. et al. Fundamental method for controlling monodisperse silica nanoparticles dimension assisted by lysine. J Sol-Gel Sci Technol 92, 134–145 (2019). https://doi.org/10.1007/s10971-019-05087-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-019-05087-z