Abstract
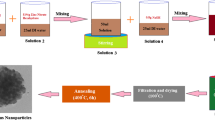
In the present research, evaporation induced self-assembly method is used to prepare nitrogen and silver co-doped mesoporous ZnO nanoparticles by means of zinc nitrate hexahydrate as a source of zinc oxide and P123 as a surface commanded medium. The physicochemical characteristics of each sample are described through several approaches. The occurrence of the wurtzite phase of ZnO along with crystallite size of the scale 22.74–22.87 nm is described by the analysis of X-ray diffraction data. Exploration of the photoluminescence and ultraviolet-visible spectrum demonstrated that the percentage rate of electron–hole recombination and band gap energy decreases continuously with the addition of nitrogen and increasing concentration of silver. Analysis of scanning electron microscope and transmission electron microscope images confirms the existence of mesoporous flocks of nanoparticles with 40–50 nm crystallite size. BET analysis has been done to determine the pore volume, surface area, and pore radius with the mesoporous nature of the samples. Analysis of FTIR spectra corroborates the existence of different functional groups. Study of the Raman spectra shows the influence of co-doping on Raman scattering of pure ZnO nanostructures. Methylene blue, congo red, and malachite green dyes are employed to examine the photocatalytic action of the samples.

Highlights
-
Evaporation induced self assembly method is used to synthesize silver and nitrogen co-doped mesoporous ZnO nanoparticles by using triblock copolymer Pluronic P123 as a surface directing agent.
-
The crystalline, mesoporous and co-doped nature of the samples is investigated by different techniques, i.e., XRD, SEM, EDX, TEM, BET, FTIR, UV-Vis, PL, and Raman spectroscopy.
-
As prepared samples describe satisfactorily the photocatalytic degradation activities against MB, MG, and CR dyes in the presence of visible light.
-
The improvement in photocatalytic activity is due to high pore volume, higher surface area, and nanoscale crystalline size with co-doping of silver and nitrogen.
























Similar content being viewed by others
References
Ban J-J, Xu G-C, Zhang L, Lin H, Sun Z-P, Lv Y, Jia D-Z (2017) Mesoporous ZnO microcube derived from a metal-organic framework as photocatalyst for the degradation of organic dyes. J Solid State Chem. https://doi.org/10.1016/j.jssc.2017.09.002
Ahmad KS, Jaffri SB (2018) Phytosynthetic Ag doped ZnO nanoparticles: semiconducting green remediators, Photocatalytic and antimicrobial potential of green nanoparticles. Open Chem. https://doi.org/10.1515/chem-2018-0060
Tripathy N, Ahmad R, KuK H, Hahn Y-B, Khang G (2016) Mesoporous ZnO nanoclusters as an ultra-active photocatalyst. Ceram Int. https://doi.org/10.1016/j.ceramint.2016.03.030
Khan MI (2017) Investigations of structural, morphological and optical properties of Cu: ZnO/TiO2/ZnO and Cu:TiO2/ZnO/TiO2 thin films prepared by spray pyrolysis technique. Results Phys. https://doi.org/10.1016/j.rinp.2017.08.038
Yatskiv R, Tiagulskyi S, Grym J (2018) Characterization of Graphite/ZnO Schottky Barriers Formed on Polar and Nonpolar ZnO Surfaces. physica status solidi (a). https://doi.org/10.1002/pssa.201800734
Yang X, Qiu L, Luo X (2018) ZIF-8 derived Ag-doped ZnO photocatalyst with enhanced photocatalytic activity. RSC Adv. https://doi.org/10.1039/C7RA13351K
Narayanana N, Deepaka NK (2018) B-N codoped p type ZnO thin films for optoelectronic applications. Mater Res. https://doi.org/10.1590/1980-5373-MR-2017-0618
Tiron I-LV, Stanescu D, Magnan H, Sirghi L (2016) High visible light photocatalytic activity of nitrogen-doped ZnO thin films deposited by HiPIMS Vasile. Surf Coat Tech. https://doi.org/10.1016/j.surfcoat.2016.11.087
Laurenti M, Cauda V (2018) Gentamicin-Releasing Mesoporous ZnO Structures. Materials. https://doi.org/10.3390/ma11020314
Li W, Wang G, Chen C, Liao J, Li Z (2017) Enhanced visible light photocatalytic activity of ZnO nanowires doped with Mn2+ and Co2+ Ions. Nanomaterials. https://doi.org/10.3390/nano7010020
Feng Y, Wang G, Liao J, Li W, Chen C, Li M, Li Z (2017) Honeycomb-like ZnO mesoporous nanowall arrays modified with Ag nanoparticles for highly efficient photocatalytic activity. Nature. https://doi.org/10.1038/s41598-017-11100-8
Li X, Peng K, Dou Y, Chen J, Zhang Y, An G (2018) Facile synthesis of Wormhole-like mesoporous tin oxide via evaporation-induced self-assembly and the enhanced gas-sensing properties. Nanoscale Res Lett. https://doi.org/10.1186/s11671-018-2434-4
Feng D, Gao T-N, Fan M, Li A, Li K, Wang T, Huo Q, Qiao Z-A (2018) A general ligand-assisted self-assembly approach to crystalline mesoporous metal oxides. NPG Asia Mater. https://doi.org/10.1038/s41427-018-0072-z
Shen Z, Zhou H, Chen H, Xu H, Feng C, Zhou X (2018) Synthesis of nano-zinc oxide loaded on mesoporous silica by coordination effect and its photocatalytic degradation property of methyl orange. J Nanomater. https://doi.org/10.3390/nano8050317
Saravanan S, Silambarasan M, Soga T (2014) Structural, morphological and optical studies of Ag-doped ZnO nanoparticles synthesized by simple solution combustion method. Japan Soc Appl Phys. https://doi.org/10.7567/JJAP.53.11RF01/meta
Bindu P, Thomas S (2014) Estimation of lattice strain in ZnO nanoparticles: X-ray peak profile analysis, J Theoretical Appl Phys. https://doi.org/10.1007/s40094-014-0141-9
Clug HP, Alexander LE (1974) Polycrystalline and amorphous materials. 2nd edition, New York
Pan JH, Zhang X, Du AJ, Bai H, Ng J, Sun D (2012) A hierarchically assembled mesoporous ZnO hemisphere array and hollow microspheres for photocatalytic membrane water filtration. Phys Chem Chem Phys. https://doi.org/10.1039/c2cp40997f
Putz AM, Len A, Ianasi C, Savii C, Almasy L (2016) Ultrasonic preparation of mesoporous silica using pyridinium ionic liquid Korean J Chem Eng 33(3):749–754. https://doi.org/10.1007/s11814-016-0021-x
Ladavos AK, Katsoulidis AP, Iosifidis A, Triantafyllidis KS, Pinnavaia TJ, Pomonis PJ (2012) Micropor Mesopors Mater. https://doi.org/10.1016/j.micromeso.2011.11.005
Goh EG, Xu X, McCormick PG (2014) Effect of particle size on the UV absorbance of zinc oxide nanoparticles. Scripta Materialia. https://doi.org/10.1016/j.scriptamat.2014.01.033
Kazeminezhad, Saadatmand S, Youselfi R (2016) Effect of transition metal elements on the structural and optical properties of ZnO nanoparticles. Bull Mater Sci. https://doi.org/10.1007/s12034-016-1206-yI
Katiyar A, Kumar N, Srivastava A (2018) Optical properties of ZnO nanoparticles synthesized by co-precipitation method using LiOH. Mater Today: Proc. https://doi.org/10.1016/j.matpr.2017.10.034
Thangeeswari T, George AT, Kumar AA (2016) Optical properties and FTIR studies of cobalt doped ZnO nanoparticles by simple solution method. Ind J Sci Technol. https://doi.org/10.17485/ijst/2016/v9i1/85776
Shia S, Xub J, Lib L (2017) Preparation and photocatalytic activity of ZnO nanorods and ZnO/Cu2O nanocomposites. Main Group Chem. https://doi.org/10.3233/MGC-160224
Zhang R, Yin P-G, Wang N, Guo L (2009) Photoluminescence and Raman scattering of ZnO nanorods, Solid State Sci. https://doi.org/10.1016/j.solidstatesciences.2008.10.016
Kumari R, Sahai A, Goswami N (2015) Effect of nitrogen doping on structural and optical properties of ZnO nanoparticles. Prog Nat Sci Mater. https://doi.org/10.1016/j.pnsc.2015.08.003
Lavand AB, Malghe YS (2015) Synthesis, characterization and visible light photocatalytic activity of nitrogen-doped zinc oxide nanospheres. J Asian Ceramic Soc. https://doi.org/10.1016/j.jascer.2015.06.002
Ahmed A, Siddique MN, Alam U, Ali T, Tripathi P (2018) Improved photocatalytic activity of Sr doped SnO2 nanoparticles: a role of oxygen vacancy. Appl Surf Sci. https://doi.org/10.1016/j.apsusc.2018.08.182
Brahmia O (2016) Photocatalytic Degradation of a Textile Dye under UV and Solar Light Irradiation Using TiO2 and ZnO nanoparticles. Int J Adv Chem Eng Biol Sci. https://doi.org/10.15242/IJACEBS.U1016204
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chauhan, N., Singh, V., Kumar, S. et al. Preparation of silver and nitrogen co-doped mesoporous zinc oxide nanoparticles by evaporation induced self assembly process to study their photocatalytic activity. J Sol-Gel Sci Technol 90, 390–403 (2019). https://doi.org/10.1007/s10971-019-04969-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-019-04969-6