Abstract
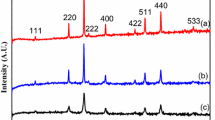
Cobalt ferrite (CoFe2O4) powders were synthesized by solution combustion method using a mixture of cetyltrimethylammonium bromide (CTAB) and glycine fuels. The combustion behavior, phase evolution, microstructure, specific surface area, and magnetic properties were investigated by thermal analysis, infrared spectroscopy, X-ray diffractometry, nitrogen adsorption–desorption, electron microscopy, and vibrating sample magnetometry techniques. Single-phase CoFe2O4 powders were achieved by mixing of CTAB and glycine fuels, regardless of fuel content, while the impurity Co3O4 and α-Fe2O3 phases together with CoFe2O4 phase were disappeared at higher amounts of CTAB fuel. Bulky microstructure of the as-combusted powders using CTAB fuel with specific surface area of 43 m2/g was transformed to foamy and porous structure (196 m2/g) by means of the mixture of CTAB and glycine fuels. Furthermore, the solution combusted CoFe2O4 powders using mixture of CTAB and glycine fuels exhibited the higher saturation magnetization due to their higher crystallinity and particle size.

The high-surface area CoFe2O4 powders were synthesized by the mixture of CTAB and glycine fuels.







Similar content being viewed by others
References
Varma A, Mukasyan AS, Rogachev AS, Manukyan KV (2016) Solution combustion synthesis of nanoscale materials. Chem Rev 116:14493–14586
Wen W, Wu J-M (2014) Nanomaterials via solution combustion synthesis: a step nearer to controllability. RSC Adv 4:58090–58100
Aruna ST, Mukasyan AS (2008) Combustion synthesis and nanomaterials. Curr Opin Solid State Mater Sci 12:44–50
Mukasyan AS, Rogachev AS, Aruna ST (2015) Combustion synthesis in nanostructured reactive systems. Adv Powder Technol 26:954–976
Manukyan KV, Chen Y-S, Rouvimov S, Li P, Li X, Dong S, Liu X, Furdyna JK, Orlov A, Bernstein GH, Porod W, Roslyakov S, Mukasyan AS (2014) Ultrasmall α-Fe2O3 superparamagnetic nanoparticles with high magnetization prepared by template-assisted combustion process. J Phys Chem C 118:16264–16271
Nersisyan HH, Lee JH, Ding J-R, Kim K-S, Manukyan KV, Mukasyan AS (2017) Combustion synthesis of zero-, one-, two- and three-dimensional nanostructures: Current trends and future perspectives. Progress Energy Combust Sci 63:79–118
Pourgolmohammad B, Masoudpanah SM, Aboutalebi MR (2017) Effect of starting solution acidity on the characteristics of CoFe2O4 powders prepared by solution combustion synthesis. J Magn Magn Mater 424:352–358
Zhang X, Jiang W, Song D, Sun H, Sun Z, Li F (2009) Salt-assisted combustion synthesis of highly dispersed superparamagnetic CoFe2O4 nanoparticles. J Alloy Compd 475:L34–L37
Parnianfar H, Masoudpanah SM, Alamolhoda S, Fathi H (2017) Mixture of fuels for solution combustion synthesis of porous Fe3O4 powders. J Magn Magn Mater 432:24–29
Fathi H, Masoudpanah SM, Alamolhoda S, Parnianfar H (2017) Effect of fuel type on the microstructure and magnetic properties of solution combusted Fe3O4 powders. Ceram Int 43:7448–7453
Chen W, Li F, Yu J, Liu L (2006) A facile and novel route to high surface area ceria-based nanopowders by salt-assisted solution combustion synthesis. Mater Sci Eng: B 133:151–156
Radpour M, Masoudpanah SM, Alamolhoda S (2017) Microwave-assisted solution combustion synthesis of Fe3O4 powders. Ceram Int 43:14756–14762
Erri P, Pranda P, Varma A (2004) Oxidizer−fuel interactions in aqueous combustion synthesis. 1. Iron(III) nitrate−model fuels. Ind Eng Chem Res 43:3092–3096
Lazarova T, Georgieva M, Tzankov D, Voykova D, Aleksandrov L, Cherkezova-Zheleva Z, Kovacheva D (2017) Influence of the type of fuel used for the solution combustion synthesis on the structure, morphology and magnetic properties of nanosized NiFe2O4. J Alloy Compd 700:272–283
Dong C, Xiao X, Chen G, Guan H, Wang Y (2015) Morphology control of porous CuO by surfactant using combustion method. Appl Surf Sci 349:844–848
Makhlouf MT, Abu-Zied BM, Mansoure TH (2014) Effect of fuel/oxidizer ratio and the calcination temperature on the preparation of microporous-nanostructured tricobalt tetraoxide. Adv Powder Technol 25:560–566
Harikrishnan V, Saravanan P, Ezhil Vizhi R, Babu DR, Vinod VTP, Kejzlar P, Černík M (2016) Effect of annealing temperature on the structural and magnetic properties of CTAB-capped SrFe12O19 platelets. J Magn Magn Mater 401:775–783
Yin W, Wang W, Zhou L, Sun S, Zhang L (2010) CTAB-assisted synthesis of monoclinic BiVO4 photocatalyst and its highly efficient degradation of organic dye under visible-light irradiation. J Hazard Mater 173:194–199
Rai AK, Gim J, Thi TV, Ahn D, Cho SJ, Kim J (2014) High rate capability and long cycle stability of Co3O4/CoFe2O4 nanocomposite as an anode material for high-performance secondary lithium ion batteries. J Phys Chem C 118:11234–11243
Li N, Zheng M, Chang X, Ji G, Lu H, Xue L, Pan L, Cao J (2011) Preparation of magnetic CoFe2O4-functionalized graphene sheets via a facile hydrothermal method and their adsorption properties. J Solid State Chem 184:953–958
Reddy DHK, Yun Y-S (2016) Spinel ferrite magnetic adsorbents: Alternative future materials for water purification? Coord Chem Rev 315:90–111
Millot N, Le Gallet S, Aymes D, Bernard F, Grin Y (2007) Spark plasma sintering of cobalt ferrite nanopowders prepared by coprecipitation and hydrothermal synthesis. J Eur Ceram Soc 27:921–926
Reddy MP, Mohamed AMA, Zhou XB, Du S, Huang Q (2015) A facile hydrothermal synthesis, characterization and magnetic properties of mesoporous CoFe2O4 nanospheres. J Magn Magn Mater 388:40–44
Houshiar M, Zebhi F, Razi ZJ, Alidoust A, Askari Z (2014) Synthesis of cobalt ferrite (CoFe2O4) nanoparticles using combustion, coprecipitation, and precipitation methods: A comparison study of size, structural, and magnetic properties. J Magn Magn Mater 371:43–48
Najmoddin N, Beitollahi A, Kavas H, Majid Mohseni S, Rezaie H, Åkerman J, Toprak MS (2014) XRD cation distribution and magnetic properties of mesoporous Zn-substituted CuFe2O4. Ceram Int 40:3619–3625
Manukyan KV, Cross A, Roslyakov S, Rouvimov S, Rogachev AS, Wolf EE, Mukasyan AS (2013) Solution combustion synthesis of nano-crystalline metallic materials: Mechanistic studies. J Phys Chem C 117:24417–24427
Socrates G (2001) Infrared and Raman characteristic group frequencies, 1 edn. Wiley, New York, NY
Nakamoto K, Nakamoto K (1977) Infrared and Raman spectra of inorganic and coordination compounds. Wiley, New York
Javadi S, Masoudpanah SM, Zakeri A (2016) Conventional versus microwave combustion synthesis of CoFe2O4 nanoparticles. J Sol-Gel Sci Technol 79:176–183
Naderi P, Masoudpanah SM, Alamolhoda S (2017) Magnetic properties of Li0.5Fe2.5O4 nanoparticles synthesized by solution combustion method. Appl Phys A 123:702
Goworek J, Kierys A, Gac W, Borówka A, Kusak R (2009) Thermal degradation of CTAB in as-synthesized MCM-41. J Therm Anal Calorim 96:375–382
Li F-t, Ran J, Jaroniec M, Qiao SZ (2015) Solution combustion synthesis of metal oxide nanomaterials for energy storage and conversion. Nanoscale 7:17590–17610
Erri P, Nader J, Varma A (2008) Controlling combustion wave propagation for transition metal/alloy/cermet foam synthesis. Adv Mater 20:1243–1245
Sickafus KE, Wills JM, Grimes NW (1999) Structure of Spinel. J Am Ceram Soc 82:3279–3292
Pourgolmohammad B, Masoudpanah SM, Aboutalebi MR (2017) Effects of the fuel type and fuel content on the specific surface area and magnetic properties of solution combusted CoFe2O4 nanoparticles. Ceram Int 43:8262–8268
Sing KSW, Everett DH, Haul RAW, Moscou L, Pierotti RA, Rouquerol J, Siemieniewska T (2008) Reporting physisorption data for gas/solid systems, handbook of heterogeneous catalysis. Wiley-VCH Verlag GmbH & Co. KgaA, Singapore
Radpour M, Alamolhoda S, Masoudpanah SM (2017) Effects of pH value on the microstructure and magnetic properties of solution combusted Fe3O4 powders. Ceram Int 43:13729–13734
Maaz K, Mumtaz A, Hasanain SK, Ceylan A (2007) Synthesis and magnetic properties of cobalt ferrite (CoFe2O4) nanoparticles prepared by wet chemical route. J Magn Magn Mater 308:289–295
Cullity BD, Graham CD (2011) Introduction to magnetic materials. Wiley, New Jersey
Spaldin NA (2010) Magnetic materials: fundamentals and applications. Cambridge University Press, New York
Ranjith Kumar E, Jayaprakash R, Prakash T (2014) The effect of annealing on phase evolution, microstructure and magnetic properties of Mn substituted CoFe2O4 nanoparticles. J Magn Magn Mater 358-359:123–127
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Highlights
-
CTAB was used as fuel in solution combustion synthesis of CoFe2O4 powders.
-
The formation of single-phase CoFe2O4 powders was facilitated using glycine accompanied by CTAB.
-
The high-surface area CoFe2O4 powders were synthesized by the mixture of CTAB and glycine fuels.
-
The higher crystallinity and particle size by the mixture of fuels lead to the higher Ms.
Rights and permissions
About this article
Cite this article
Famenin Nezhad Hamedani, S., Masoudpanah, S.M., Bafghi, M.S. et al. Solution combustion synthesis of CoFe2O4 powders using mixture of CTAB and glycine fuels. J Sol-Gel Sci Technol 86, 743–750 (2018). https://doi.org/10.1007/s10971-018-4671-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-018-4671-5