Abstract
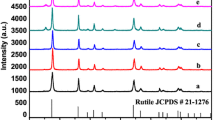
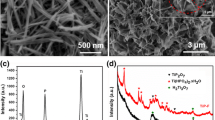
The synthesis of nanocrystals with hierarchical structure is a well-known challenge in many fields of science and technology. In the present work, we making use of tetrabutyl titanate as a titanium source, hydrofluoric acid as an additive, successfully prepared flaky flower titanium dioxide (TiO2) with sperical nanostructures by the solvothermal method. Then we calcined some of the samples. It was found that the calcined sample presented higher crystallinity by X-ray diffraction (XRD). A series of scanning electron microscopy (SEM) characterization results indicate that the calcination causes the sheet-like structure of the sample to become larger and thicker. And the exposed specific surface area became larger. The Brunauer–Emmett–Teller N2 gas adsorption–desorption isotherms characterization results also further demonstrate that the calcined samples have a larger specific surface area than the sample before calcined. The photocatalytic activity was evaluated by measuring the degradation rate of methylene blue under UV–vis light irradiation. The results show that anatase TiO2 after calcination exhibit higher photocatalytic activity than the sample before calcination in the degradation of methylene blue under UV–vis light irradiation. It can be attributed to the synergetic effect of the architecture, high crystallinity, large specific surface areas and ostwald ripening.
Graphical abstract
Scheme 1. Schematic illustration of the morphological evolution. 









Similar content being viewed by others
References
Kudo A, Miseki Y (2009) Chem Soc Rev 38:253–278
Zhang HJ, Chen GH, Bahnemann DW (2009) J Mater Chem 19:5089–5121
Fujishima A, Rao TN, Tryk DA (2000) J Photochem Photobiol C 1:1–21
Subramanian A, Karki KI, Gnanasekar FP, Eddy B, Rambabu J (2006) Power Sources 159:186–192
Wu NL, Wang SY, Rusakova IA (1999) Science 285:1357–1377
Gratzel M (2001) Nature 414:338–344
Wang R, Cai X, Shen FL (2013) Ceram Int 39:9465–9470
Khan SUM, Al - Shahry M, Ingler Jr. WB (2002) Science 297:2243–2245
Li YX, Lu GX, Li SB (2001) Appl Catal A Gen 214:179
Wang TH, Li YX, Peng SQ, Lu GX, Li SB (2005) Acta Chim Sin 63:797–801
Li YX, Xie CF, Peng SQ, Lu GX, Li SBJ (2008) Mol Catal A Chem 282:117–123
Xu SH, Feng DL, Li DX, Shangguan WF (2008) Chin J Inorg Chem 24:785–790
Li FB, Li XZ, Hou MF, Cheah KW, Choy WCH (2005) Appl Catal A Gen 285:181–189
Yu JG, Yu JC, Leung MKP, Ho WK, Cheng B, Zhao XJ, Zhao JC (2003) J Catal 255(2):309–320
Yun J, Jin D, Lee YS, Kim H (2010) Mater Lett 64:2431–2434
Kim MH, Baik JM, Zhang JP, Larson C, Li YL, Stucky GD, Moskovits M, Wodtke AM (2010) J Phys Chem C 114:10697–10702
Zhang XG, Ge X, Wang C (2009) Cryst Growth Des 9:4301–4307
Allam NK, El - Sayed MA (2010) J Phys Chem C 114:12024–12029
Allam NK, Shankar K, Grimes CA (2008) J Mater Chem 18:2341–2348
Ho JY, Huang MH (2009) J Phys Chem C 113:14159–14164
Wu GS, Wang JP, Thomas DF, Chen AC (2008) Langmuir 24:3503–3509
Ban T, Nakatani T, Uehara Y, Ohya Y (2008) Cryst Growth Des 8:935–940
Hosono E, Fujihara S, Imai H, Honma I, Masaki I, Zhou HS (2007) ACS Nano 1:273–278
Dai SY, Wang KJ, Wu QC (1997) Acta Energiae Solaris Sinica 18(2):228–232
Dai XJ, Luo YS, Zhang WD, Fu SY (2010) Dalton Trans 39:3426–3432
Wang CX, Yin LW, Zhang LY, Qi YX, Lun N, Liu NN (2010) Langmuir 26:12841–12848
Zhuang ZB, Peng Q, Liu JF, Wang X, Li YD (2007) Inorg Chem 46:5179–5187
Xie JS, Wu QS, Zhang D, Ding YP (2009) Cryst Growth Des 9:3889–3897
Zhou YX, Yao HB, Zhang YQ, Gong JY, Liu SJ, Yu SH (2009) Inorg Chem 48:1082–1090
Hu XL, Yu JC, Gong JM (2007) J Phys Chem C 111:11180–11185
Ma C, Moore D, Li J, Wang ZL (2003) Adv Mater 15:228–231
Jiang XC, Wang YL, Herricks T, Xia YN (2004) J Mater Chem 14:695–703
Lee JC, Kim TG, Choi HJ, Sung YM (2007) Cryst Growth Des 7:2588–2593
Bian ZF, Zhu J, Cao FL, Lu YF, Li HX (2009) Chem Commun 25(25):3789–3791
Feng JY, Yin MC, Wang ZQ, Yan SC, Wan LJ, Li ZS, Zou ZG (2010) CrystEngComm 12:3425–3429
Chen JS, Tan YL, Li CM et al. (2010) J Am Chem Soc 132(17):6124–6130
Xiang QJ, Yu JG (2011) Chin J Catal 32(3):525–531
Yu JC, Yu JG, Ho WK, Jiang ZT, Zhang LZ (2002) Chem Mater 14:3808–3816
Tian GH, Chen YJ, Zhou W, Pan K, Tian CG, Huang XR, Fu HG (2011) Crystengcomm 13:2994–3000
Ren HT, Yang Q (2017) Appl Surf Sci 396:530–538
Acknowledgements
We acknowledge the financial supports of the National Natural Science Foundation (No. 51402157, 51602164) and project supported by the outstanding scientific research innovation team plan for university of Shandong province, P. R. China.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Zhang, L., Liu, H., Lv, S. et al. Synthesis and photocatalysis of flaky flower TiO 2 with sphere structure. J Sol-Gel Sci Technol 84, 283–289 (2017). https://doi.org/10.1007/s10971-017-4493-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-017-4493-x