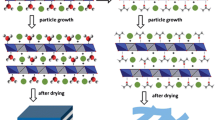
Abstract
A new type of hierarchically porous architecture of Mg2+, Al3+-containing layered double hydroxide, with well defined mesoporous/macroporous structure, has been fabricated by dry gel conversion method without the need of a surfactant and organic solvent. Predefined amorphous aluminum hydroxide gel and anhydrous magnesium sulfate were used as Al and Mg precursor material, respectively, and aqueous ammonia as precipitating agent. The resulting self-sustaining hierarchical layered double hydroxide material exhibits a bi-modal porous structure having a macroporous network with macropore sizes of 100–200 nm and a well defined mesoporous structure of pore size around 3.9 nm in the macroporous framework. Layered double hydroxide crystallites are aligned with a multilayer manner to form the architecture structure. The formation process of layered double hydroxide architecture, such as the evolution of phase composition, pore structure, and particle morphology with reaction time was further verified and the formation mechanism is postulated.
Graphical Abstract







Similar content being viewed by others
References
Vaccari A (2001) Layered double hydroxides: present and future. Nova, Inc, New York, NY
Braterman PS, Xu ZP, Yarberry F (2004) Handbook of Layered Materials. Marcel Dekker, New York, NY
Williams GR, O’Hare D (2006) Towards understanding, control and application of layered double hydroxide chemistry. J Mater Chem 16:3065–3074
Evans DG, Slade RCT (2006) Structural aspects of layered double hydroxides. Springer Berlin Heidelberg. Struct Bond 119:1–87
Wang Q, O’Hare D (2012) Recent advances in the synthesis and application of layered double hydroxide (LDH) nanosheets. Chem Rev 112:4124–4155
Kuang Y, Zhao L, Zhang S et al. (2010) Morphologies, preparations and applications of layered double hydroxide micro-/nanostructures. Materials 3:5220–5235
Gunawan P, Xu R (2008) Synthesis of unusual coral-like layered double hydroxide microspheres in a nonaqueous polar solvent/surfactant system. J Mater Chem 18:2112–2120
Prevot V, Caperaa N, Taviot-Gueho C et al. (2009) Glycine-assisted hydrothermal synthesis of NiAl-layered double hydroxide nanostructures. Cryst Growth Des 9:3646–3654
Shao M, Ning F, Zhao J et al. (2013) Hierarchical layered double hydroxide microspheres with largely enhanced performance for ethanol electrooxidation. Adv Funct Mater 23:3513–3518
Li L, Ma R, Iyi N et al. (2006) Hollow nanoshell of layered double hydroxide. Chem commun 42:3125–3127
Gunawan P, Xu R (2009) Direct assembly of anisotropic layered double hydroxide (LDH) nanocrystals on spherical template for fabrication of drug-LDH hollow nanospheres. Chem Mater 21:781–783
Li L, Feng Y, Li Y et al. (2009) Fe3O4 core/layered double hydroxide shell nanocomposite: versatile magnetic matrix for anionic functional materials. Angew Chem 121:6002–6006
Géraud E, Prévot V, Ghanbaja J et al. (2006) Macroscopically ordered hydrotalcite-type materials using self-assembled colloidal crystal template. Chem mater 18:238–240
Géraud E, Rafqah S, Sarakha M et al. (2008) Three dimensionally ordered macroporous layered double hydroxides: preparation by templated impregnation/coprecipitation and pattern stability upon calcination. Chem Mater 20:1116–1125
Whitesides GM, Grzybowski B (2002) Self-assembly at all scales. Science 295:2418–2421
Xu L, Ma W, Wang L et al. (2013) Nanoparticle assemblies: dimensional transformation of nanomaterials and scalability. Chem Soc Rev 42:3114–3126
Chang CC, Cho HJ, Wang Z et al. (2015) Fluoride-free synthesis of a Sn-BEA catalyst by dry gel conversion. Green Chem 17:2943–2951
Chen B, Huang Y (2011) Formation of microporous material AlPO4-18 under dry-gel conversion conditions. Microporous Mesoporous Mater 143:14–21
Naik SP, Chiang AST, Thompson RW (2003) Synthesis of zeolitic mesoporous materials by dry gel conversion under controlled humidity. J Phys Chem B 107:7006–7014
Matsukata M, Ogura M, Osaki T et al. (1999) Conversion of dry gel to microporous crystals in gas phase. Top Catal 9:77–92
Xu W, Dong J, Li J et al. (1990) A novel method for the preparation of zeolite ZSM-5. J Chem Soc Chem Commun 10:755–756
Matsufuji T, Nishiyama N, Ueyama K et al. (2000) Permeation characteristics of butaneisomers through MFI-type zeolitic membranes. Catal Today 56:265–273
Alfaro S, Arruebo M, Coronas J et al. (2001) Preparation of MFI type tubular membranes by steam-assisted crystallization. Microporous Mesoporous Mater 50:195–200
Shi Q, Chen Z, Song Z et al. (2011) Synthesis of ZIF-8 and ZIF-67 by steam-assisted conversion and an investigation of their tribological behaviors. Angew Chem Int Ed 50:672–675
Meng X, Xiao FS (2014) Green routes for synthesis of zeolites. Chem Rev 114:1521–1543
Möller K, Yilmaz B, Jacubinas RM et al. (2011) One-step synthesis of hierarchical zeolite beta via network formation of uniform nanocrystals. J Am Chem Soc 133:5284–5295
Han SW, Kim J, Ryoo R (2017) Dry-gel synthesis of mesoporous MFI zeolite nanosponges using a structure-directing surfactant. Microporous Mesoporous Mater 240:123–129
Li H, Jin J, Wu W et al. (2011) Synthesis of a hierarchically macro-/mesoporous zeolite based on a micro-emulsion mechanism. J Mater Chem 21:19395–19401
Goergen S, Guillon E, Patarin J, Rouleau L (2009) Shape controlled zeolite EU-1 (EUO) catalysts: dry gel conversion type synthesis, characterization and formation mechanisms. Microporous Mesoporous Mater 126:283–290
Kloprogge JT, Wharton D, Hickey L, Frost RL (2002) Infrared and Raman study of interlayer anions CO3 2−, NO3 −, SO4 2− and CIO4 − in Mg/Al-hydrotalcite. Am Mineral 87:623–629
Faour A, Mousty C, Prevot V et al. (2012) Correlation among structure, microstructure, and electrochemical properties of NiAl-CO3 layered double hydroxide thin films. J Phys Chem C 116:15646–15659
Chen HY, Zhang FZ, Chen T, Xu SL, Evans DG, Duan X (2009) Comparison of the evolution and growth processes of films of M/Al-layered double hydroxides with M=Ni or Zn. Chem Eng Sci 64:2617–2622
Yamaguchi N, Ando D, Tadanaga K, Tatsumisago M (2007) Direct formation of Mg-Al-layered double hydroxide films on glass substrate by the sol-gel method with hot water treatment. J Am Ceram Soc 90:1940–1942
Nguyen T, Boudard M, Carmezim MJ, Montemor MF (2017) NixCo1-x(OH)2 nanosheets on carbon nanofoam paper as high areal capacity electrodes for hybrid supercapacitors. Energy 126:208–216
Guo XX, Zhang FZ, Evans DG, Duan X (2010) Layered double hydroxide films: synthesis, properties and applications. Chem Commun 46:5197–5210
Fogg AM, Williams GR, Chester R, O’Hare D (2004) A novel family of layered double hydroxides-[MAl4(OH)12](NO3)2·xH2O (M=Co, Ni, Cu, Zn). J Mater Chem 14:2369–2371
Seron A, Delorme F (2008) Synthesis of layered double hydroxides (LDHs) with varying pH: a valuable contribution to the study of Mg/Al LDH formation mechanism. J Phys Chem Solids 69:1088–1090
Yang YM, Zhao XF, Zhu Y, Zhang FZ (2012) Transformation mechanism of magnesium and aluminum precursor solution into crystallites of layered double hydroxide. Chem Mater 24:81–87
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 21376019, 21676013), the Fundamental Research Funds for the Central Universities (No. YS1406), and Beijing Engineering Center for Hierarchical Catalysts.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Jingjing Han and Rong Zhang authors are contributed equally to this work.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Han, J., Zhang, R., Zhang, Y. et al. Template-free fabrication of hierarchically meso/macroporous architecture of layered double hydroxide by dry gel conversion method. J Sol-Gel Sci Technol 83, 609–617 (2017). https://doi.org/10.1007/s10971-017-4466-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-017-4466-0