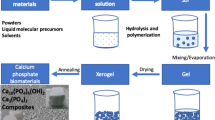
Abstract
In this research, high-pure monticellite ceramic (CaMgSiO4) nanoparticles were synthesized via a novel sol–gel method using ethanol and chloride ions for the acceleration of polycondensation process. The gel obtained from metal alkoxide and metal salts precursors dried at 100°C; then, the dried-gel calcined at different temperatures to monitor the structural development of the final samples. The effect of various heat-treatment temperatures on the x-ray diffraction patterns, followed by the calculations from scherrer’s equation, showed that the grain size of the synthesized samples at 1200°C was around 28 nm. Based on dynamic light scattering spectroscopy, the size of most of the particles was ~85 nm. Williamson–Hall formula was also used to calculate the lattice strain of the samples. According to the results, by changing the heat-treatment temperature, lattice strain/stress, lattice parameters and grain size have meaningfully changed. The proposed synthesis method showed superior advantages in comparison with the other conventional techniques presented in the literature, which is relatively faster at lower temperatures. The synthesized high-pure monticellite nanoparticles could be applied in different biomedical engineering applications in which the purity and structural properties are of importance, such as dental, bone tissue engineering, coatings on biomedical implants, surgery hemostasis and inducing osteogenesis in vivo applications.
Graphical abstract











Similar content being viewed by others
References
Colombo P (2008) In praise of pores. Science 322(5900):381–383
Dapporto M, Sprio S, Fabbi C, Figallo E, Tampieri A (2016) A novel route for the synthesis of macroporous bioceramics for bone regeneration. J Eur Ceram Soc 36(9):2383–2388
Deville S (2008) Freeze-casting of porous ceramics: a review of current achievements and issues. Adv Eng Mater 10(3):155–169
Ohji T, Fukushima M (2012) Macro-porous ceramics: processing and properties. Int Mater Rev 57(2):115–131
Mozafari M (2014) Bioceramics in the realm of history. Bioceram Dev Appl 4:e106
Fukui H, Ohsuka H, Hino T, Kanamura K (2010) A Si−O−C Composite Anode: High Capability and Proposed Mechanism of Lithium Storage Associated with Microstructural Characteristics. ACS Appl Mater Interfaces 2(4):998–1008
Saadati MR, Maleki A, Niroumand B, Allafchian AR (2016) A novel low cost method for the synthesis of ceramic nano silicon oxycarbide powder. Ceram Int 42(7):8531–8536
Zamanian A, Yasaei M, Ghaffari M, Mozafari M (2013) Calcium hydroxide-modified zinc polycarboxylate dental cements. Ceram Int 39(8):9525–9532
Azami M, Jalilifiroozinezhad S, Mozafari M, Rabiee M (2011) Synthesis and solubility of calcium fluoride/hydroxy-fluorapatite nanocrystals for dental applications. Ceram Int 37(6):2007–2014
Dutta S, Passi D, Singh P, Bhuibhar A (2015) Ceramic and non-ceramic hydroxyapatite as a bone graft material: a brief review. Ir J Med Sci 184(1):101–106. (1971)
Ramedani A, Yazdanpanah A, Moztarzadeh F, Mozafari M (2014) On the use of nanoliposomes as soft templates for controlled nucleation and growth of hydroxyapatite nanocrystals under hydrothermal conditions. Ceram Int 40(7):9377–9381
Ghaffari M, Moztarzadeh F, Sepahvandi A, Mozafari M, Faghihi S (2013) How bone marrow-derived human mesenchymal stem cells respond to poorly crystalline apatite coated orthopedic and dental titanium implants. Ceram Int 39(7):7793–7802
Dietrich E, Oudadesse H, Lucas‐Girot A, Mami M (2009) In vitro bioactivity of melt‐derived glass 46S6 doped with magnesium. J Biomed Mater Res A 88(4):1087–1096
Diba M, Goudouri O-M, Tapia F, Boccaccini AR (2014) Magnesium-containing bioactive polycrystalline silicate-based ceramics and glass-ceramics for biomedical applications. Curr Opin Solid St M 18(3):147–167
Webster TJ, Ergun C, Doremus RH, Bizios R (2002) Hydroxylapatite with substituted magnesium, zinc, cadmium, and yttrium. II. Mech osteoblast Adhes J Biomed Mater Res 59(2):312–317
Liu C, Yeh J, Aloia J (1988) Magnesium directly stimulates osteoblast proliferation. J Bone Miner Res 3:S104
Wu C, Chang J (2004) Synthesis and apatite-formation ability of akermanite. Mater Lett 58(19):2415–2417
Wu C, Chang J, Wang J, Ni S, Zhai W (2005) Preparation and characteristics of a calcium magnesium silicate (bredigite) bioactive ceramic. Biomaterials 26(16):2925–2931
Hafezi-Ardakani M, Moztarzadeh F, Rabiee M, Talebi AR (2011) Synthesis and characterization of nanocrystalline merwinite (Ca 3 Mg (SiO 4) 2) via sol–gel method. Ceram Int 37(1):175–180
Chen X, Ou J, Kang Y, Huang Z, Zhu H, Yin G, Wen H (2008) Synthesis and characteristics of monticellite bioactive ceramic. J Mater Sci Mater Med 19(3):1257–1263
Yazdanpanah A, Kamalian R, Moztarzadeh F, Mozafari M, Ravarian R, Tayebi L (2012) Enhancement of fracture toughness in bioactive glass-based nanocomposites with nanocrystalline forsterite as advanced biomaterials for bone tissue engineering applications. Ceram Int 38(6):5007–5014
Razavi M, Fathi M, Savabi O, Beni BH, Vashaee D, Tayebi L (2014) Surface microstructure and in vitro analysis of nanostructured akermanite (Ca 2 MgSi 2 O 7) coating on biodegradable magnesium alloy for biomedical applications. Colloid Surf B 117:432–440
Mozafari M, Moztarzadeh F, Vashaee D, Tayebi L (2012) Effects of heat treatment on physical, microstructural and optical characteristics of PbS luminescent nanocrystals. Phys E 44(7):1429–1435
Ganjali M, Pourhashem S, Mozafari M (2015) The effect of heat-treatment on the structural characteristics of nanocrystalline chlorapatite particles synthesized via an in situ wet-chemical route. Ceram Int 41(10):13100–13104
Warren BE (1969) X-ray Diffraction. Courier Corporation, New York
Dieter GE (1988) Mechanical metallurgy. McGraw-Hill Book Company, UK, vol SI Metric edn
Mozafari M, Moztarzadeh F, Tahriri M (2010) Investigation of the physico-chemical reactivity of a mesoporous bioactive SiO 2–CaO–P 2 O 5 glass in simulated body fluid. J Non-Cryst Sol 356(28):1470–1478
Iwata NY, Lee G-H, Tsunakawa S, Tokuoka Y, Kawashima N (2004) Preparation of diopside with apatite-forming ability by sol–gel process using metal alkoxide and metal salts. Colloid Surf B 33(1):1–6
Sing K, Everett D, Haul R, Moscou L, Pierotti R, Rouquerol J, Siemieniewska T (1985) Physical and biophysical chemistry division commission on colloid and surface chemistry including catalysis. Pure Appl Chem 57(4):603–619
Lowell S, Shields JE, Thomas MA, Thommes M (2012) Characterization of porous solids and powders: surface area, pore size and density, vol 16. Springer Science & Business Media, Dordrecht
Rouquerol J, Rouquerol F, Llewellyn P, Maurin G, Sing KS (2013) Adsorption by powders and porous solids: principles, methodology and applications. Academic press, Oxford, UK
Taibi M, Ammar S, Jouini N, Fiévet F, Molinié P, Drillon M (2002) Layered nickel hydroxide salts: synthesis, characterization and magnetic behaviour in relation to the basal spacing. J Mater Chem 12(11):3238–3244
Li K, Fan J, Shang M, Lian H, Lin J (2015) Sr 2 Y 8 (SiO 4) 6 O 2: Bi 3+/Eu 3+: a single-component white-emitting phosphor via energy transfer for UV w-LEDs. J Mater Chem C 3(38):9989–9998
Niu H, Yang Q, Tang K, Xie Y (2006) Large-scale synthesis of single-crystalline MgO with bone-like nanostructures. J Nanopart Res 8(6):881–888
Imtiaz A, Farrukh MA, Khaleeq-ur-Rahman M, Adnan R (2013) Micelle-assisted synthesis of Al2O3·CaO nanocatalyst: optical properties and their applications in photodegradation of 2, 4, 6-Trinitrophenol. Sci World J. doi:10.1155/2013/641420
Acknowledgements
This research was supported by Iran University of Science and Technology (Department of Nanotechnology and Chemistry). The authors would like to gratefully acknowledge Mr. Milad Khalili from Iran University of Science and Technology (IUST) for his contribution to this research.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Kalantari, E., Naghib, S.M., Reza Naimi-Jamal, M. et al. Green solvent-based sol–gel synthesis of monticellite nanoparticles: a rapid and efficient approach. J Sol-Gel Sci Technol 84, 87–95 (2017). https://doi.org/10.1007/s10971-017-4461-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-017-4461-5