Abstract
The influence of the hydration and drying process on the line shape and signal intensity of the electron paramagnetic resonance spectra recorded from Cu(II) ions present in silica xerogels calcined at various temperatures was investigated. The experimental Cu(II) electron paramagnetic resonance spectra were found to consist of a superimposition of three individual subspectra (Γ1, Γ2 and Φ), which reflect different local environments in which the Cu(II) ions were located. The results demonstrate that: (i) Within experimental error, the spin Hamiltonian parameters of each individual subspectrum remain, in the course of the experiments, identical. (ii) The hydration process changed the relative contribution from the individual subspectra (Γ1, Γ2 and Φsignificantly, and increased the overall electron paramagnetic resonance signal intensity by a factor of more than ten, as compared with the non-hydrated silica xerogels. (iii) On re-drying the hydrated silica xerogel samples, the original line shape and original signal intensity values were restored. Thus, measurement of the relative contributions of the individual subspectra can be used as a sensitive method with which to monitor the hydration/drying process in silica xerogels. As a caveat, we conclude that the influence of the hydration/drying process should be taken into account in the interpretation of Cu(II) electron paramagnetic resonance spectra of calcined silica xerogel samples, which provides the real novelty of the present report.
Graphical Abstract
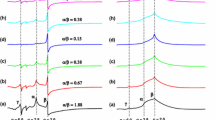
Relative contribution of subspectra (Γ1, Γ2 and Φ) to Cu(II) EPR spectra of silica xerogels calcined at the temperatures quoted and then hydrated for 15 min and for 3 days. 





Similar content being viewed by others
References
Brinker CJ, Scherer GW (1990) Sol-gel science. Academic, New York, NY
Hench LL, West JK (1990) Chem Rev 90:33–72
Hench LL, Ulrich DR (1984) Ultrastructure processing of ceramics, glasses and composites. Wiley, New York, NY
Wong J, Angel C (1976) Glass structure by spectroscopy. Marcel Dekker, Basel
Griscom DL (1990) Glass Science Technology. Academic, Boston
Tominaga H, Ono Y, Keii T (1975) J Catal 40:197–202
Darab JG, MacCrone RK (1987) J Non Cryst Solids 95, 96:1203–1210
Darab JG, MacCrone RK (1991) Phys Chem Glasses 32:91–101
Ikoma S, Tanako S, Nomoto E, Yokoi H (1989) J Non Cryst Solids 113:130–136
Klonkowski AM, Schlaepfer CW (1991) J Non Cryst Solids 129:101–108
Klonkowski AM, Schlaepfer CW (1992) J Non Cryst Solids 149:189–195
Klonkowski AM, Koehler K, Schlaepfer CW (1993) J Mater Chem 3:105–110
Shames A, Lev O, Iosofzon B (1993) J Non Cryst Solids 163:105–114
Liška M, Hulínová H, Mazur M, Pelikán P (1992) Glass & Ceramic [Sklar & Keramik] 42:247–250
Hulínová H, Liška M, Pelikán P, Mazur M (1992) Glass & Ceramic [Sklar & Keramik] 42:251–252
Pelikán P, Mazur M, Liška M, Šimurka P (1995) Curr Trends Coord Chem 2:31–36
Mazur M, Valko M, Klement R, Pelikán P (1997) Prog Coord Organomet Chem 3:285–290
Mazur M, Moncol J, Kleinová M, Stachová P, Valko M (2006) Phys Chem Glasses 47:278–282
Bogomolova LD, Pavlushkina TK, Morozova IV (2006) Glass Ceram 63:254–258
Kledzik K, Jamrógiewicz M, Gwiazda M, Wagner-Wysiecka E, Jezierska J, Biernat JF, Klonkowski AM (2007) Mater Sci Pol 25:1041–1051
Vignali F, Predieri G, Feci E, Palanti S, Baratto MC, Basosi R, Callone E, Müller K (2011) J Solgel Sci Technol 60:445–456
Sivasubramanian G, Shanmugam C, Parameswaran VR (2013) J Porous Mater 20:417–430
Mazur M, Husáriková L, Valko M, Rhodes CJ (2016) Appl Magn Reson 47:1–12
Mazur M, Švorec J, Kleinová M, Valko M (2003) Prog Coord Bioinorg Chem 6:317–322
Breyer T, Breitbarth FW, Vogelsberger W (2003) J Colloid Interface Sci 266:153–159
Liška M, Mazur M, Hulínová H, Pelikán P, Valko M, Nerád I (1995) Ceram Silic 39:69–72
Mazur M, Valko M, Klement R, Morris H (1996) Anal Chim Acta 333:249–252
Mazur M, Valko M, Morris H, Klement R (1996) Anal Chim Acta 333:253–265
Thiele H, Etstling J, Such P, Hoefer P (1992) WinEPR. Bruker Analytic Gmb, Berlin
Weber RT (1995) WinEPR SimFonia. EPR Division, Bruker Instr. Inc, Billerica, MA
Pelikán P, Liška M, Valko M, Mazur M (1996) J Magn Reson 122:9–15
Shames A, Lev O, Iosefzon-Kuyavskaya B (1994) J Non Cryst Solids 175:14–20
Dyrek K, Che M (1997) Chem Rev 97:305–331
Dyrek K, Adamski A, Sojka Z (1998) Spectrochem Acta A 54:2337–2348
Mazur M, Valko M (2002) Phys Chem Glass 43:237–240
Mazur M, Kleinová M, Moncol J, Stachová P, Valko M, Telser J (2006) J Non Cryst Solids 352:3158–3165
Mazur M, Husáriková L, Rhodes CJ, Valko M (2015) J Solgel Sci Technol 76:110–119
Dexmer J, Leroy CM, Binet L, Heresanu V, Launois P, Steunou N, Coulon C, Maquet J, Brun N, Livage J, Backov R (2008) Chem Mater 20:5541–5549
Riou D, Roubeau O, Bouhedja L, Livage J, Ferey G (2000) Chem Mater 12:67–72
Kahn A, Livage J, Collongues R (1974) Phys Status Solidi 26:175–179
Nedelec JM, Bouazaoui M, Turrell S (1999) Phys Chem Glass 40:264–268
Martini G, Bassetti V (1979) J Phys Chem 89:2505–2511
Martini G, Bassetti V (1979) J Phys Chem 89:2511–2515
Bassetti V, Burlamacchi L, Martini G (1979) J Phys Chem 101:5471–5477
Kawashima M, Matsu KJ (1999) Ceram Soc Jpn 107:282–284
Kawashima M, Oda N, Uchida Y, Matsu K (2000) Lumin J 87–89:685–687
Kawashima M, Oda N, Uchida Y, Matsu KJ (2002) Ceram Soc Jpn 110:507–511
Abiddi N, Deroide B, Zanchetta JV, Bourret D, Elmkami H, Rumori P (1996) Phys Chem Glasses 37:149–154
Abiddi N, Deroide B, Zanchetta JV (1997) Nucleonika 42:505–514
Rumori P, Deroide B, Abiddi N, Zanchetta JV (1997) J Non Cryst Solids 221:59–69
Klonkowski AM, Widernik T, Grobelna B, Jóźwiak WK, Prog aH, Szubiakiewicz E (2001) J Solgel Sci Technol 20:161–180
Klonkowski AM, Grobelna B, Widernik T, Jankowska-Frydel A, Mozgawa W (1999) Langmuir 15:5814–5819
Klonkowski AM, Koehler K, Widernik T, Grobelna B (1996) J Mater Chem 6:579–584
Acknowledgements
This work was supported by the Slovak Research and Development Agency under the contact No. APVV-15-0053 and by the Scientific Grant Agency of the Slovak Republic (Projects VEGA 1/0041/15 and VEGA 1/0686/17).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mazur, M., Valko, M. & Rhodes, C.J. A systematic study of the hydration and drying process of silica xerogels using Cu(II) EPR spectroscopy. J Sol-Gel Sci Technol 82, 855–861 (2017). https://doi.org/10.1007/s10971-017-4357-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-017-4357-4