Abstract
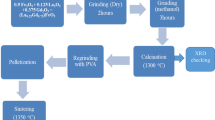
Ceramic powder of BaCe0.54Zr0.36Y0.1O2.95 (BCZY) was successfully synthesized via a modified sol–gel method using metal nitrate salts as precursors. The synthesis was accomplished by using three different types of surfactants which are cationic (benzalkonium chloride), anionic (sodium dodecyl sulfate) and a nonionic surfactant (polyoxyethylene (10) oleyl ether). Citric acid and ethylene glycol were used as a chelating and a polymerization agent, respectively. The crystal form and morphology of the powders were characterized by Fourier transform infrared (FTIR) spectroscopy, X-ray diffractometer and scanning electron microscope (SEM). FTIR spectra showed the traces of carbonate residues in all samples due to the presence of hydrocarbon group in the surfactant structure even after calcination process at T = 1100 °C. Samples prepared using cationic and anionic surfactant consists of the multi-phases compounds which are dominated by BaCO3, BaCeO3, CeO2 and BaZrO3. On the other hand, the samples prepared by using nonionic surfactants produce a single phase of BCZY perovskite-type oxide. SEM images revealed that the sample prepared without surfactant exhibits severe agglomeration. Morphology of the particles for the BCZY prepared by applying the cationic and anionic surfactant was, respectively, cubical and spherical in shape. As for nonionic surfactant, the particle obtained was spherical and uniform in shape. The optimum result was obtained by adding a nonionic surfactant, Brij97, which indicates high crystallinity of the BCZY powder at a temperature of 950 °C and the particle size ranging from 20 to 80 nm. It can be concluded that surfactant affects the phase formation of BCZY ceramic powder as well as its morphology.
Graphical Abstract









Similar content being viewed by others
References
Barison S, Fabrizio M, Fasolin S, Montagner F, Mortalò C (2010) Mater Res Bull 45(9):1171–1176
Iwahara H (1995) Solid State Ion 77:289–298
Yang K, Wang JX, Xue YJ, Wang MS, He CR, Wang Q, Miao H, Wang WG (2014) Ceram Int 40(9):15073–15081
Chakroborty A, Sharma AD, Maiti B, Maiti HS (2002) Mater Lett 57:862–867
Wang S, Zhao F, Zhang L, Chen F (2012) Solid State Ion 213:29–35
Li Y, Zhao J, Wang B (2004) Mater Res Bull 39(3):365–374
Abdullah NA, Hasan S, Osman N (2013) J Chem 2013:1–7
Osman N, Jani AM, Talib IA (2007) Ionics 12(6):379–384
Kobayashi Y, Iizuka Y, Tanase T, Konno M (2005) J Sol-Gel Sci Tech 33:315–321
Emami S, Hosseini HRM, Dolati A (2012) Trans Nonferrous Met Soc China 53(4):308–314
Huang GY, Xu SM, Li LY, Wang XJ (2014) Trans Nonferrous Met Soc China 24:3739–3746
Cioatera N, Pârvulescu V, Su BL (2010) Mater Chem Phys 120(2–3):697–701
Wang YD, Ma CL, Sun XD, Li HD (2002) Inorg Chem Commun 5:751–755
Chandradass J, Kim KH (2009) J Cryst Growth 311(14):3631–3635
Graeve OA, Fathi H, Kelly JP, Saterlie MS, Sinha K, Rojas-George G, Kanakala R, Brown DR, Lopez EA (2013) J Colloid Interf Sci 407:302–309
Wang Y, Wang C, Li C, Cheng Y, Chi F (2014) Ceram Int 40(3):4305–4310
Tao Y, Shao J, Wang J, Wang WG (2009) J Alloy Compd 484(1–2):729–733
Abdullah NA, Osman S, Hasan H, Hassan OH (2012) Int J Electrochem Sc 7:9401–9409
Ejehi F, Marashi SPH, Ghaani MR, Haghshenas DF (2012) Ceram Int 38(8):6857–6863
Motta M, Deimling CV, Saeki MJ, Lisboa-Filho PN (2008) J Sol-Gel Sci Tech 46(2):201–207
Osman N, Talib IA, Hamid HA (2010) Ionics 16(6):561–569
Kuo WK, Lo B, Ling YC (1999) Mater Chem Phys 60(2):132–136
Namnam JS, Philip J (2012) J Colloid Interf Sci 366(1):88–95
Guan H, Bestland E, Zhu C, Zhu H, Albertsdottir D, Hutson J, Simmons T, Ginic-Markovic M, Tao X, Ellis AV (2010) J Hazardous Mater 183:616–621
Khomane RB, Agrawal AC, Kulkarni BD, Gopukumar S, Sivashanmugam A (2008) Mater Res Bull 43(8–9):2497–2503
Abu Bakar SN (2010) Abu Talib I, Osman N. World Appl Sci 9:26–28
Cizauskaite S, Reichlova V, Nenartaviciene G, Beganskiene A, Pinkas J, Kareiva A (2007) Mater Sci 25(3):755–765
Osman N, Talib IA, Hamid HA (2009) Sains Malays 38(3):401–405
Liu Y, Guo Y, Ran R, Shao Z (2013) J Membrane Sci 437:189–195
Robert CL, Ansart F, Castillo S, Richard G (2002) Solid State Sci 4:1053–1059
Kumari L, Li WZ, Kulkarni S, Wu KH, Chen W, Wang C, Vannoy CH, Leblanc RM (2009) Nanoscale Res Lett 5(1):149–157
Lin XF, Zhou RM, Zhang JQ, Sheng XH (2010) Mater Sci 28(2):503–511
Zhang S, Jiang F, Qu G, Lin C (2008) Mater Lett 62(15):2225–2228
Wang M, Gao Y, Dai L, Cao C, Guo X (2012) J Solid State Chem 189:49–56
Wang Z, Li X, Feng Z (2011) Bull Korean Chem Soc 32(4):1310–1314
Rai P, Song MK, Song HM, Kim JH, Kim YS, Lee IH, Yu YT (2012) Ceram Int 38:235–242
Acknowledgments
The authors would like to thank the Minister of Higher Education for the Research Grant 600-RMI/RAGS 5/3 (1/2012), Fundamental Research Grant 600-RMI/FRGS 5/3 (8/2014) and Universiti Teknologi MARA (UiTM) for facilities and supports.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mazlan, N.A., Osman, N., Md Jani, A.M. et al. Role of ionic and nonionic surfactant on the phase formation and morphology of Ba(Ce,Zr)O3 solid solution. J Sol-Gel Sci Technol 78, 50–59 (2016). https://doi.org/10.1007/s10971-015-3938-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-015-3938-3