Abstract
We prepared the catechol-functionalized nanosilica for adsorption of germanium (Ge) ions from aqueous media. The functionalized nanosilica was characterized by FTIR, TGA, TEM, BET, and XPS. The influence of pH, Ge initial concentration, and time of the adsorption process was investigated. Under the optimum condition, the maximum adsorption capacity of Ge ions was 0.048 mg m−2 at pH 4.5. The adsorption mechanism is the chelating interaction between hydroxyl and Ge(OH)4. The functionalized nanosilica was able to selectively adsorb Ge ions from tellurium(IV) and boron. Langmuir isotherm was found to better fit the experiment data rather than Freundlich isotherm. The kinetics of the adsorption process of Ge ions on the functionalized nanosilica was investigated using the pseudo-first-order and pseudo-second-order models, and the results indicated that the pseudo-second order equation model provided the best correlation with the experimental results.
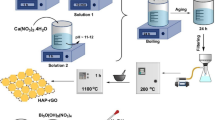
Graphical Abstract












Similar content being viewed by others
References
Bernstein LR (1985) Germanium geochemistry and mineralogy. Geochim Cosmochim Acta 49(11):2409–2422
Gao HW, Liu WG (2000) Spectrophotometric investigation of germanium complex solution with o-chlorophenylfluorone and determination of trace amounts of germanium. Bull Korean Chem Soc 21(11):1090–1094
Brown RD (2004) Minerals yearbook, vol I. Metals and minerals. U.S. Geological Survey, Washington, DC
Kalderis D, Tsolaki E, Antoniou C, Diamadopoulos E (2008) Characterization and treatment of wastewater produced during the hydro-metallurgical extraction of germanium from fly ash. Desalination 230:162–174
Cote G, Bauer D (1980) Liquid–liquid extraction of germanium with oxine derivatives. Hydrometallurgy 5(2):149–160
Schepper AD (1976) Liquid–liquid extraction of germanium by LIX 63. Hydrometallurgy 1:291–298
Tutkun O, Demircan N, Kumbasar RA (1999) Extraction of germanium from acidic leach solutions by liquid membrane technique. Clean Technol Environ Policy 1(2):148–153
Inukai Y, Tanaka Y, Shiraishi Y, Matsuda T, Mihara N, Yamada K et al (2001) Selective separation of germanium(IV) by di(2-hydroxyethyl)amine-type cellulose derivative. Anal Sci 1:1117–1120
Yasuda S, Kawazu K (1991) Separation of germanium from ethylene glycol distillates by N-methylglucamine resin. Sep Sci Technol 26(9):1273–1277
Harada A, Tarutani T, Yoshimura K (1988) Spectrophotometric determination of germanium in rocks after selective adsorption on sephadex gel. Anal Chim Acta 209:333–338
Pokrovski GS, Martin F, Hazemann JL, Schott J (2000) An X-ray adsorption fine structure spectroscopy study of germanium-organic ligand complexes in aqueous solution. Chem Geol 163:151–165
Marco-Lozar JP, Linares-Solano A, Cazorla-Amorós D (2011) Effect of the porous texture and surface chemistry of activated carbons on the adsorption of a germanium complex from dilute aqueous solutions. Carbon 49(10):3325–3331
Yasuda S, Inukai Y, Ohba H (1994) Adsorption behaviors of germanium(IV) on the 1,2-propanediol type resins. J Chem Soc Jpn Chem Ind Chem Nippon Kagaku Kaishi 3:211–213
Inukai Y, Kaida Y, Yasuda S (2001) Selective separation of germanium(IV) by iminodiacetic acid-type chitosan chelating resin. Rep Kyushu Natl Ind Res Inst 13(3):339–344
Göktürk G, Delzendeh M, Volkan M (2000) Preconcentration of germanium on mercapto-modified silica gel. Spectrochim Acta Part B 55:1063–1071
Wang J, Zheng S, Shao Y, Liu J, Xu Z, Zhu D (2010) Amino-functionalized Fe3O4@SiO2 core-shell magnetic nanomaterial as a novel adsorbent for aqueous heavy metals removal. J Colloid Interface Sci 349(1):293–299
Wang C, Zhang L, Guo Z, Xu J, Wang H, Zhai K (2010) A novel hydrazine electrochemical sensor based on the high specific surface area grapheme. Microchim Acta 169(1–2):1–6
Lei Z, Xingjia G, Hongmei L, Zhu Y, Xueyan L, Tianci X (2011) Separation of trace amounts of Ga and Ge in aqueous solution using nano-particles micro-column. Talanta 85(5):2463–2469
Bennett GF (2004) Adsorbents: fundamentals and Applications: Ralph T. Yang, John Wiley & Sons, Hoboken, NJ, 2003, US$ 94.95, 422 pp., ISBN: 0-471-29741-0. J Hazard Mater 109(1–3):227–228
Du JJ, Yuan YP, Sun JX, Peng FM, Jiang X, Qiu LG (2011) New photocatalysts based on MIL-53 metal-organic frameworks for the decolorization of methylene blue dye. J Hazard Mater 190:945–951
Madrakian T, Afkhami A, Zolfigol MA, Ahmadi M, Koukabi N (2012) Application of modified silica coated magnetite nanoparticles for removal of iodine from water samples. Micro Nano Lett 4(1):57–63
Shen XC, Fang XZ, Zhou YH, Hong L (2004) Synthesis and characterization of 3-aminopropyltriethoxysilane-modified superparamagnetic magnetite nanoparticles. Chem Lett 33(11):1468–1469
Gao J, Liu F, Liu Y, Liu Y, Ma N, Wang Z, Zhang X (2010) Environment-friendly method to produce graphene that employs vitamin C and amino acid. Chem Mater 22(7):2213–2218
Stankovich S, Dikin DA, Piner RD, Kohlhaas KA, Kleinhammes A, Jia Y (2007) Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 45(7):1558–1565
Si W, Wu X, Jin Z, Guo F, Zhuo S, Cui H (2013) Reduced graphene oxide aerogel with high-rate supercapacitive performance in aqueous electrolytes. Nanoscale Res Lett 8(1):1–8
Inukai Y, Kaida Y, Yasuda S (1997) Selective adsorbents for germanium(IV) derived from chitosan. Anal Chim Acta 343(3):275–279
Marco-Lozar JP, Cazorla-Amorós D, Linares-Solano A (2007) A new strategy for germanium adsorption on activated carbon by complex formation. Carbon 45(13):2519–2528
Pokrovski GS, Schott J (1998) Experimental study of the complexation of silicon and germanium with aqueous organic species: implications for germanium and silicon transport and Ge/Si ratio in natural waters. Geochim Cosmochim Acta 62(21–22):3413–3428
Liang J, Fan L, Xu K, Huang Y (2012) Study on extracting of germanium with trioctylamine. Energy Procedia 17:1965–1973
Fan HT, Sun XT, Zhang ZG, Li WX (2014) Selective removal of lead(II) from aqueous solution by an ion-imprinted silica sorbent functionalized with chelating N-donor atoms. J Chem Eng 59(6):2106–2114
Kul AR, Koyuncu H (2010) Adsorption of Pb(II) ions from aqueous solution by native and activated bentonite: kinetic, equilibrium and thermodynamic study. J Hazard Mater 179(1–3):332–339
Kyzas GZ, Siafaka PI, Pavlidou EG, Chrissafis KJ, Bikiaris DN (2015) Synthesis and adsorption application of succinyl-grafted chitosan for the simultaneous removal of zinc and cationic dye from binary hazardous mixtures. Chem Eng J 259:438–448
Inukai Y, Kaida Y, Yasuda S (2001) Selective separation of germanium(IV) by iminodiacetic acid-type chitosan chelating resin. Rep Kyushu Natl Ind Res Inst 13(3):4055–4060
Yasuda S, Inukai Y, Ohba H (1994) Adsorption behaviors of germanium(IV) on the 1,2-propanediol type resins. J Chem Soc Jpn Chem Ind Chem Nippon Kagaku Kaishi 1994:211–213
Acknowledgments
The authors are grateful for the financial support from the National Natural Science Foundation (No. 51464024), Applied Basic Research Projects in Yunnan Province (2013FB096), and Young and Middle-aged Academic Technology Leader Backup Talent Cultivation Program in Yunnan Province (2012HB008).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cui, W., Wang, S., Peng, J. et al. Catechol-functionalized nanosilica for adsorption of germanium ions from aqueous media. J Sol-Gel Sci Technol 77, 666–674 (2016). https://doi.org/10.1007/s10971-015-3898-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-015-3898-7