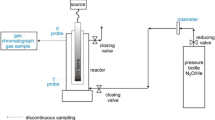
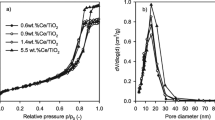
Abstract
TiO2 photocatalyst was prepared unconventionally, using the sol–gel process controlled within the reverse micelles and the processing by pressurized hot fluids as an alternative to standard calcination. Conventional calcined TiO2 was prepared as well. Textural, microstructural and optical properties of prepared photocatalysts were characterized by using nitrogen physisorption, powder X-ray diffraction and DR UV–Vis spectroscopy. The photocatalytic properties of developed TiO2 catalysts were investigated in the photocatalytic reduction of CO2 and photocatalytic decomposition of N2O. It was revealed that TiO2 processed by pressurized hot fluids shows significantly improved textural properties and different crystallinity compared to its calcined analog. Yields of both reaction products (H2 and CH4) of CO2 photoreduction were higher for the extracted TiO2 photocatalyst. The same result was achieved in N2O photodecomposition. The maximum N2O conversion (83 % after 20 h of illumination) in inert gas was reached also over the TiO2 extracted photocatalyst, and it can be attributed to simultaneous N2O photocatalytic decomposition and N2O photolysis. Reaction kinetics of N2O-photoinduced decomposition was described well by the pseudo-first-rate law. The surface heterojunction of bicrystalline anatase–brookite phase corresponded to better catalytic activity of TiO2 processed by pressurized hot fluids in both reactions, in a consequence of reduced electron–hole pair recombination.
Graphical Abstract






Similar content being viewed by others
References
Li K, An X, Parka KH, Khraishehb M, Tang J (2014) A critical review of CO2 photoconversion: catalysts and reactors. Catal Today 224:3–12
Richter R, Caillol S (2011) Fighting global warming: the potential of photocatalysis against CO2, CH4, N2O, CFCs, tropospheric O3, BC and other major contributors to climate change. J Photochem Photobiol C Photochem Rev 12(1):1–19
Richter R, Kiesgen R, Tingzhen M, Caillol S (2013) Fighting global warming by photocatalytic reduction of CO2 using giant photocatalytic reactors. Renew Sustain Energy Rev 19:82–106
Habisreutinger SN, Schmidt-Mende L, Stolarczyk JK (2013) Photocatalytic reduction of CO2 on TiO2 and other semiconductors. Angew Chem Int Ed 52:7372–7408
Wang W, Wang S, Ma X, Gong J (2011) Recent advances in catalytic hydrogenation of carbon dioxide. Chem Soc Rev 40:3703–3727
Maeda K, Domen K (2010) Photocatalytic water splitting: recent progress and future challenges. J Phys Chem Lett 1:2655–2661
Benson EE, Kubiak CP, Sathrum AJ, Smieja JM (2009) Electrocatalytic and homogeneous approaches to conversion of CO2 to liquid fuels. Chem Soc Rev 38:89–99
Sano T, Negishi N, Mas D, Takeuchi K (2000) Photocatalytic decomposition of N2O on highly dispersed Ag+ ions on TiO2 prepared by photodeposition. J Catal 194:71–79
Cheng H, Wang J, Zhao Y, Han X (2014) Effect of phase composition, morphology, and specific surface area on the photocatalytic activity of TiO2 nanomaterials. RCS Adv 4:47031–47038
Stafford U, Gray KA, Kamat PV, Varma A (1993) An insitu diffuse reflectances FTIR investigation of photocatalytic degradation of 4-chlorphenol on a TiO2 powder surface. Chem Phys Lett 205:55–61
Hurum DC, Agrios AG, Gray KA, Rajh T, Thurnauer MC (2003) Explaining the enhanced photocatalytic activity of Degussa P25 mixed-phase TiO2 using EPR. J Phys Chem B 107:4545–4549
Linsebigler AL, Lu G, Yates JT (1995) Photocatalysis on TiO2 surfaces—principles, mechanisms and selected results. Chem Rev 95:735–758
Su R, Bechstein R, So L, Vang RT, Sillassen M, Esbjornsson B, Palmqvist A, Besenbacher F (2011) How the anatase-to-rutile ratio influences the photoreactivity of TiO2. J Phys Chem C 115:24287–24292
Li SX, Chen J, Zheng FY, Li YC, Huang FY (2013) Synthesis of the double-shell anatase-rutile TiO2 hollow spheres with enhanced photocatalytic activity. Nanoscale 5:12150–12155
Jing DL, Zhang SQ, Zhao HJ (2007) Photocatalytic degradation characteristics of different organic compounds at TiO2 nanoporous film electrodes with mixed anatase/rutile phases. Environ Sci Technol 41:303–308
Kawahara T, Ozawa T, Iwasaki M, Tada H, Ito S (2003) Photocatalytic activity of rutile–anatase coupled TiO2 particles prepared by a dissolution–reprecipitation method. J Colloid Interface Sci 267:377–381
Rui ZB, Wu SR, Peng C, Ji HB (2014) Comparison of TiO2 Degussa P25 with anatase and rutile crystalline phases for methane combustion. Chem Eng J 243:254–264
Lee J, Sorescu DC, Deng X (2011) Electron-induced dissociation of CO2 on TiO2(110). J Am Chem Soc 133:10066–10069
Tan S, Zhao Y, Zhao J, Wang Z, Ma C, Zhao A, Wang B, Luo Y, Yang J, Hou J (2011) CO2 dissociation activated through electron attachment on the reduced rutile TiO2(110)-1x1 surface. Phys Rev B 84:155418–155427
Tanaka K, White JM (1982) Dissociative adsorption of CO2 on oxidized and reduced Pt/TiO2. J Phys Chem 86:3977–3980
Thomas AG, Syres KL (2012) Adsorption of organic molecules on rutile TiO2 and anatase TiO2 single crystal surfaces. Chem Soc Rev 41:4207–4217
Koci K, Obalova L, Matejova L, Placha D, Lacny Z, Jirkovsky J, Solcova O (2009) Effect of TiO2 particle size on the photocatalytic reduction of CO2. Appl Catal B 89:494–502
Liu L, Zhao H, Andino JM, Li Y (2012) Photocatalytic CO2 reduction with H2O on TiO2 nanocrystals: comparison of anatase, rutile, and brookite polymorphs and exploration of surface chemistry. ACS Catal 2:1817–1828
Chen L, Graham M, Li G, Gentner D, Dimitrijevic N, Gray K (2009) Photoreduction of CO2 by TiO2 nanocomposites synthesized through reactive direct current magnetron sputter deposition. Thin Solid Films 517:5641–5645
Kočí K, Krejčíková S, Lacný Z, Šolcová O, Obalová L (2012) Photocatalytic decomposition of N2O on Ag–TiO2. Catal Today 191:134–137
Luttrell T, Halpegamage S, Tao J, Kramer A, Sutter E, Batzill M (2014) Why is anatase a better photocatalyst than rutile?—Model studies on epitaxial TiO2 films. Sci Rep 4:1–5
Jing L, Li S, Song S, Xue L, Fu H (2008) Investigation on the electron transfer between anatase and rutile in nano-sized TiO2 by means of surface photovoltage technique and its effects on the photocatalytic activity. Sol Energy Mater Sol Cells 92:1030–1036
Sun B, Smirniotis PG (2003) Interaction of anatase and rutile TiO2 particles in aqueous photooxidation. Catal Today 88:49–59
Li G, Chen L, Graham ME, Gray KA (2007) A comparison of mixed phase Titania photocatalysts prepared by physical and chemical methods: the importance of the solid–solid interface. J Mol Catal A 275:30–35
Matějová L, Cajthaml T, Matěj Z, Benada O, Klusoň P, Šolcová O (2010) Super/subcritical fluid extractions for preparation of the crystalline titania. J Supercrit Fluids 52:215–221
Matějová L, Matěj Z, Fajgar R, Cajthaml T, Šolcová O (2012) TiO2 powders synthesized by pressurized fluid extraction and supercritical drying: effect of water and methanol on structural properties and purity. Mater Res Bull 47:3573–3579
Gregg SJ, Sing KSW (1982) Adsorption, surface area, and porosity. Academic Press, New York
Brunauer S, Emmett PH, Teller E (1938) Adsorption of gases in multimolecular layers. J Am Chem Soc 60:309–319
Schneider P (1995) Adsorption isotherms of microporous-mesoporous solids revisited. Appl Catal A 129:157–259
DeBoer JB, Lippens BC, Linsen BG, Broekhoff JCP, Heuvel AVD, Osinga ThJ (1966) The t-curve of multimolecular N2-adsorption. J Colloid Interface Sci 21:405–414
Lecloux A, Pirard JP (1979) The importance of standard isotherms in the analysis of adsorption isotherms for determining the porous texture of solids. J Colloid Interface Sci 70:265–281
Barret EP, Joyner LG, Halenda PB (1951) The determination of pore volume and area distributions in porous substances. I. Computations from nitrogen isotherms. J Am Chem Soc 73:373–380
Scardi P, Leoni M (2002) Whole powder pattern modelling. Acta Crystallogr Sect A: Found Crystallogr 58:190–200
Matěj Z, Kužel R (2009) MStruct—program/library for MicroStructure analysis by powder diffraction. http://www.xray.cz/mstruct/. Accessed 22 July 2015
Matej Z, Kuzel R, Nichtova L (2010) XRD total pattern fitting applied to study of microstructure of TiO2 films. Powder Diffr 25:125–131
Matěj Z, Matějová L, Kužel R (2013) XRD analysis of nanocrystalline anatase powders prepared by various chemical routes: correlations between micro-structure and crystal structure parameters. Powder Diffr 28:161–183
Kočí K, Matějová L, Kozák O, Čapek L, Valeš V, Reli M, Praus P, Šafářová K, Kotarba A, Obalová L (2014) ZnS/MMT nanocomposites: the effect of ZnS loading in MMT on the photocatalytic reduction of carbon dioxide. Appl Catal B 158–159:410–417
Ballari MM, Brandi R, Alfano O, Cassano A (2008) Mass transfer limitations in photocatalytic reactors employing titanium dioxide suspensions—I. Concentration profiles in the bulk. Chem Eng J 136:50–65
Ballari MM, Brandi R, Alfano O, Cassano A (2008) Mass transfer limitations in photocatalytic reactors employing titanium dioxide suspensions—II. External and internal particle constrains for the reaction. Chem Eng J 136:242–255
Kočí K, Obalová L, Plachá D, Lacný Z (2008) Effect of temperature, pressure and volume of reacting phase on photocatalytic CO2 reduction on suspended nanocrystalline TiO2. Collect Czech Chem Commun 73:1192–1204
Kočí K, Reli M, Kozák O, Lacný Z, Plachá D, Praus P, Obalová L (2011) Influence of reactor geometry on the yield of CO2 photocatalytic reduction. Catal Today 176:212–214
Liu L, Pitts DT, Zhao H, Zhao C, Li Y (2013) Silver-incorporated bicrystalline (anatase/brookite) TiO2 microspheres for CO2 photoreduction with water in the presence of methanol. Appl Catal A General 467:474–482
Kandiel TA, Feldhoff A, Robben L, Dillert R, Bahnemann DW (2010) Tailored titanium dioxide nanomaterials: anatase nanoparticles and brookite nanorods as highly active photocatalysts. Chem Mater 22:2050–2060
Gai LG, Duan XQ, Jiang HH, Mei QH, Zhou GW, Tian Y, Liu H (2012) One-pot synthesis of nitrogen-doped TiO2 nanorods with anatase/brookite structures and enhanced photocatalytic activity. Cryst Eng Comm 14:7662–7671
Li JG, Ishigaki T, Sun XD (2007) Anatase, brookite, and rutile nanocrystals via redox reactions under mild hydrothermal conditions: phase-selective synthesis and physicochemical properties. J Phys Chem C 111:4969–4976
Boppella R, Basak P, Manorama SV (2012) Viable method for the synthesis of biphasic TiO2 nanocrystals with tunable phase composition and enabled visible-light photocatalytic performance. ACS Appl Mater Interfaces 4:1239–1246
Zhao HL, Liu LJ, Andino JM, Li Y (2013) Bicrystalline TiO2 with controllable anatase–brookite phase content for enhanced CO2 photoreduction to fuels. J Mater Chem A 28:8209–8216
Liu L, Li Y (2014) Understanding the reaction mechanism of photocatalytic reduction of CO2 with H2O on TiO2-based photocatalysts: a review. Aerosol Air Qual Res 14:453–469
Anpo M, Yamashita H, Ichihashi Y, Ehara S (1995) Photocatalytic reduction of CO2 with H2O on various titanium oxide catalysts. J Electroanal Chem 396:21–26
Li GH, Gray KA (2007) The solid–solid interface: explaining the high and unique photocatalytic reactivity of TiO2-based nanocomposite materials. Chem Phys 339:173–187
He H, Liu C, Dubois KD, Jin T, Louis ME, Li G (2012) Enhanced charge separation in nanostructured TiO2 materials for photocatalytic and photovoltaic applications. Ind Eng Chem Res 51:11841–11849
Zhang Y, Gan H, Zhang G (2011) A novel mixed-phase TiO2/kaolinite composites and their photocatalytic aktivity for degradation of organic contaminants. Chem Eng J 172:936–943
Matějová L, Kočí K, Reli M, Čapek L, Hospodková A, Peikertová P, Matěj Z, Obalová L, Wach A, Kustrowski P, Kotarba A (2014) Preparation, characterization and photocatalytic properties of cerium doped TiO2: on the effect of Ce loading on the photocatalytic reduction of carbon dioxide. Appl Catal B 152–153:172–183
Zhao B, Chen F, Jiao Y, Yang H, Zhang J (2011) Ag0-loaded brookite/anatase composite with enhanced photocatalytic performance towards the degradation of methyl orange. J Mol Catal A: Chem 348:114–119
Yan MC, Chen F, Zhang JL, Anpo M (2005) Preparation of controllable crystalline Titania and study on the photocatalytic properties. J Phys Chem B 109:8673–8678
Yu JC, Zhang L, Yu JG (2002) Direct sonochemical preparation and characterization of highly active mesoporous TiO2 with a bicrystalline framework. Chem Mater 14:4647–4653
Acknowledgments
The financial support of the Grant Agency of the Czech Republic (Projects Nos. 14-23274S and 14-35327J), the Taiwan NSC-103-2923-E-002-009-MY3, and the EU project “ENET” (No. CZ.1.05/2.1.00/03.0069) is gratefully acknowledged. Authors also thank to the support of the “National Feasibility Program I” (project LO1208 “TEWEP”) from Ministry of Education, Youth and Sports of the Czech Republic.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Kočí, K., Matějová, L., Obalová, L. et al. Preparation, characterization and photocatalytic performance of TiO2 prepared by using pressurized fluids in CO2 reduction and N2O decomposition. J Sol-Gel Sci Technol 76, 621–629 (2015). https://doi.org/10.1007/s10971-015-3813-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-015-3813-2