Abstract
Efficient rice-shaped polyaniline@bismuth oxide (PANI@Bi2O3) nanocomposites were synthesized by in situ sol–gel method. The sol of polyaniline was added to the gel of Bi2O3 in different mixing volume ratios of inorganic reactant and with a fixed mixing volume ratio, i.e., 1:1, of organic polymer. The physicochemical characterization was carried out by SEM, XRD, FTIR, UV–Vis and simultaneous TGA studies. PANI@Bi2O3 were deposited on a silver electrode (AgE, surface area, 0.0216 cm2) to give an efficient sensor with a fast response toward the toxic 3-methoxy phenol in liquid phase. The calibration plot is linear (r 2 = 0.9809) over the 0.09 nM to 0.09 mM 3-methoxy phenol concentration ranges. The sensitivity is ~0.8796 µAcm−2 µM−1, and the detection limit is 0.016 ± 0.002 nM (signal-to-noise ratio, at a SNR of 3), which leads to a promising future sensitive sensor development using organic–inorganic nanocomposites by reliable I–V method for the potential applications of hazardous phenolic compounds in environmental and healthcare fields.
Graphical Abstract
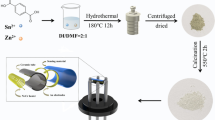
Schematic diagram for the synthesis of rice-shaped polyaniline@Bi2O3 nanocomposite.








Similar content being viewed by others
References
Usuki A, Kojima M, Okada A, Fukushima Y, Kamigaito T (1993) Synthesis of nylon 6-clay hybrid. J Mater Res 8:1179–1184
Kojma Y, Usuki A, Kawasumi M, Fukushima Y, Okada A, Kurayuchi T, Kamigaito O (1993) Mechanical-properties of nylon 6-clay hybrid. J Mater Res 8:1185–1189
Liu J, Tanaka T, Sivula K, Alivisatos AP, Fréchet JMJ (2004) Employing end-functional polythiophene to control the morphology of nanocrystal-polymer composites in hybrid solar cells. J Am Chem Soc 126:6550–6551
Gangopadhyay R, De A (2010) Conducting polymer nanocomposites: a brief overview. Chem Mater 12:608–622
Tian ZQ, Lian YZ, Wang JQ, Wang SJ, Li WH (1991) Electrochemical and XPS studies on the generation of silver clusters in polyaniline films. J Electroanal Chem Interfacial Electrochem 308:357–363
Drelinkiewicz A, Hasik M, Kloc M (2000) Pd/polyaniline as the catalysts for 2-ethylanthraquinone hydrogenation. The effect of palladium dispersion. Catal Lett 64:41–47
Gangopadhyay R, De A (2000) Conducting polymer nanocomposites: a brief overview. Chem Mater 12:608–622
Khan SB, Lee J-W, Marwani HM, Akhtar K, Asiri AM, Seo J et al (2014) Polybenzimidazole hybrid membranes as selective adsorbent of mercury. Compos Part B Eng 56:392–396
Rahman MM, Khan SB, Asiri AM, Marwani HM, Qusti AH (2013) Selective detection of toxic Pb(II) ions based on wet-chemically prepared nanosheets integrated CuO–ZnO nanocomposites. Compos Part B Eng 54:215–223
Khan SB, Alamry KA, Marwani HM, Asiri AM, Rahman MM (2013) Synthesis and environmental applications of cellulose/ZrO2 nanohybrid as a selective adsorbent for nickel ion. Compos Part B Eng 50:253–258
Pillalamarri SK, Blum FD, Tokuhiro AT, Bertino MF (2005) One-pot synthesis of polyaniline-metal nanocomposites. Chem Mater 17:5941–5944
Fujii S, Matsuzawa S, Nakamura Y, Ohtaka A, Teratani T, Akamatsu K et al (2010) Synthesis and characterization of polypyrrole–palladium nanocomposite coated latex particles and their use as a catalyst for suzuki coupling reaction in aqueous media. Langmuir 26:6230–6239
Khan A, Khan AAP, Asiri AM, Rahman MM, Alhogbi BG (2015) Preparation and properties of novel sol-gel derived electrical conducting poly (n-methyl pyrrole)Sn(II)SiO3/CNTs composite. J Solid State Electrochem 19:1479–1489
Khan A, Khan AAP, Asiri AM (2015) Preparation and characterization of hybrid graphene oxide composite and its application in paracetamol microbiosensor. Polym Compos 36:221–228
Khan A, Khan AAP, Asiri AM, Rub MA, Azum N (2014) A new way of synthesis nanohybrid cation-exchanger applicable for membrane electrode. Polym Compos 35:1436–1443
Khan A, Khan AAP, Asiri AM, Rub MA, Azum N, Khan SB, Marwani HM (2014) Applied poly(2-methoxy aniline) Sn(II)silicate carbon nanotubes composite: synthesis, characterization, structure–property relationships and applications. J Ind Eng Chem 20:2301–2309
Khan A, Khan AAP, Asiri AM, Rub MA, Azum N (2014) Dual nature, self oxidized poly(o-anisidine) functionalized multiwall carbon nanotubes composite: preparation, thermal and electrical studies. Compos Part B 58:451–456
Khan A, Khan AAP, Asiri AM, Rub MA, Azum N, Khan I (2014) Preparation, electrical conductivity and thermal studies on silver doped polyaniline phosphotungstate nanocomposite. Synth React Inorg Metal-Org Nano-Metal Chem 44:1526–1530
Khan A, Khan AAP, Asiri AM, Rub MA, Azum N, Khan SB, Rahman MM, Khan I (2013) Synthesis, characterization of silver nanoparticle embedded polyaniline tungstophosphate-nanocomposite cation exchanger and its application for heavy metal selective membrane. Compos Part B 45:1486–1492
Tiwari DC, Jain R, Sharma S (2008) Electrochemical behaviour of pyridostigmine bromide-an anticolinergic drug at polyaniline/polypyrrole composite polymer electrode Indian. J Chem Technol 15:319–324
Oriero DA, Gyan IO, Bolshaw BW, Cheng IF, Aston DE (2015) Electrospun biocatalytic hybrid silica–PVA-tyrosinase fiber mats for electrochemical detection of phenols. Microchem J 118:166–175
Karim F, Fakhruddin ANM (2012) Recent advances in the development of biosensors for phenols: a review. Rev Environ Sci Biotechnol 11:261–274
Rajesh W, Takashima K (2004) Kaneto, Amperometric phenol biosensor based on covalent immobilization of tyrosinase onto an electrochemically prepared novel copolymer of (N-3-aminopropyl pyrrole-co-pyrrole) film. Sens Actuators, B 102:271–277
Balasundram N, Sundram K, Samman S (2006) Phenolic compounds in plants and agriindustrial byproducts: antioxidant activity, occurrence, and potential uses. Food Chem 99:191–203
Yang J, Lee J, Choi S (2009) Tyrosinase-immobilized biosensor based on the functionalized hydroxyl group-MWNT and detection of phenolic compounds in red wines. J Sens 916515:1–9
Arecchi A, Scampicchio M, Drusch S, Mannino S (2010) Nanofibrous membrane based tyrosinase-biosensor for the detection of phenolic compounds. Anal Chem Acta 659:133–136
Ronkainen NJ, Halsall HB, Heineman WR (2010) Electrochemical biosensors. Chem Soc Rev 39:1747–1763
Kimmel DW, LeBlanc G, Meschievitz ME, Cliffel DE (2012) Electrochemical sensors and biosensors. Anal Chem 84:685–707
Duval C (1963) Inorganic thermogravimetric analysis. Elsevier, Amsterdam, p 315
Wang YD, Rubner MF (1990) Stability studies of the electrical-conductivity of various poly(3-alkylthiophenes). Synth Metal 39:153–175
Wang JX (1996) Mechanism of mediation of the electrochemical oxidation of K4Fe(CN)6 at poly-[tris(3-{omega-[4-(2,2’-bipyridyl)]alkyl}-thiophene)iron(II)]-filmmodified electrodes in aqueous solutions. Electrochim Acta 41:2563–2569
Roncoli J (1992) Conjugated poly(thiophenes): synthesis, functionalization, and applications. Chem Rev 92:711–738
Toshima N, Hara S (1995) Direct synthesis of conducting polymers from simple monomers. Prog Polym Sci 20:155–183
Rahman MM, Khan SB, Asiri AM (2014) Fabrication of smart chemical sensors based on transition-doped-semiconductor nanostructure materials with µ-chips. PLoS ONE 9:e85036
Cheng XL, Zhao H, Huo LH, Gao S, Zhao JG (2004) Sens Actuator B 102:248
Srivastava JK, Pandey P, Mishra VN, Dwivedi R (2001) J Natur Gas Chem 20:179
Rahman MM, Khan SB, Asiri AM (2015) A microchip based fluoride sensor based on the use of CdO doped ferric oxide nanocubes. Microchim Acta 182:487–494
Rahman MM, Khan SB, Asiri AM (2013) Chemical sensor development based on polycrystalline gold electrode embedded low-dimensional Ag2O nanoparticles. Electrochim Acta 112:422–430
Rahman MM, Jamal A, Khan SB, Faisal M (2011) Fabrication of highly sensitive ethanol chemical sensor based on Sm-doped Co3O4 nano-kernel by solution method. J Phys Chem C 115:9503–9510
Rahman MM, Jamal A, Khan SB, Faisal M (2011) Cu-doped ZnO based nanostructured materials for sensitive chemical sensor applications. ACS Appl Mater Interfaces 3:1346–1351
Rahman MM, Khan SB, Asiri AM, Alamry KA, Khan AAP, Khan A, Rub MA, Azum N (2013) Acetone sensor based on solvothermally prepared ZnO doped with Co3O4 nanorods. Microchim Acta 180:675–685
Rahman MM, Jamal A, Khan SB, Faisal M, Asiri AM (2012) Highly sensitive methanol chemical sensor based on undoped silver oxide nanoparticles prepared by a solution method. Microchim Acta 178:99–106
Rahman MM, Khan SB, Gruner G, Al-Ghamdi MS, Daous MA, Asiri AM (2013) Chloride Ion Sensors based on low-dimensional α-MnO2–Co3O4 nanoparticles fabricated glassy carbon electrodes by simple I–V technique. Electrochim Acta 103:143–150
Rahman MM, Jamal A, Khan SB, Faisal M (2011) Highly sensitive ethanol chemical sensor based on Ni-doped SnO2 nanostructure materials. Biosens Bioelectron 28:127–134
Acknowledgments
The authors are thankful for the Centre for Excellence of Advanced Materials Research (CEAMR), King Abdulaziz University, Saudi Arabia, for providing research assistance.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Khan, A., Khan, A.A.P., Rahman, M.M. et al. Toward designing efficient rice-shaped polyaniline@bismuth oxide nanocomposites for sensor application. J Sol-Gel Sci Technol 76, 519–528 (2015). https://doi.org/10.1007/s10971-015-3802-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-015-3802-5