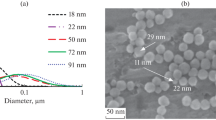
Abstract
Acoustic and electroacoustic spectroscopy were use to model varying concentrations of NH4Cl in a 25 wt% fumed silica suspension for interpretation and visualization of the critical coagulation concentration (CCC) needed to induce coagulation of the suspension. The acoustic data indicates a range for the CCC that agrees with Derjaguin and Landau, Verwey and Overbeek theory and experimental results found in literature. The electroacoustic data suggest a power law relationship between NH4Cl concentration and changes in the colloidal vibrational current, and predicts that 0.4M is the CCC for NH4Cl in a fumed silica suspension.
Graphical Abstract











Similar content being viewed by others
References
Bhandarkar S (2004) Sol-gel processing for optical communication technology. J. Am. Ceram. Soc 87:1180–1199
Costa L, Kerner D (2003) High purity glass forms by a colloidal sol-gel process. J Solgel Sci Technol 26:63–66
Teoli D, Realdon N, Guglielmi M, Rosato A, Morpurgo M, Parisi L (2006) Wet sol-gel derived silica for controlled release of proteins. J Control Release 116:295–303
Bergenholtz J, Fuchs M (1999) Gel transitions in colloidal suspensions. J Phys Condens Matter 11:10171–10182
Schmidt H, Goedicke S, Jonschker G, Mennig M (2000) The sol-gel process as a basic technology for nanoparticle-dispersed inorganic-organic composites. J Solgel Sci Technol 19:31–51
Bhattacharya S, Kieffer J (2008) Molecular dynamics simulation study of growth regimes during polycondensation of silicic acid: from silica nanoparticles to porous gels. J Phys Chem C 112:1764–1771
Brunet F, Dubois M, Cabane B, Perly B (1991) Sol-gel polymerization studied through 29Si NMR with polarization transfer. J Phys Chem 95:944–951
Pereira JCG, Price G, Catlow CRA, Almeida R (1997) Atomistic modeling of silica based sol-gel processes. J Solgel Sci Technol 8:55–58
Charpentier AP, Li X, Sui R (2009) Study of the sol-gel reaction mechanism in supercritical co2 for the formation of sio2 nanocomposites. Langmuir 25:3748–3754
Shea KJ, Loy DA (2001) A mechanistic investigation of gelation. The sol-gel polymerization of precursors to bridged polysilsesquioxanes. Acc Chem Res 34:707–716
Sink K (2010) Influence of chemical conditions on the nanoporous structure of silicate aerogels. Materials 3:704–740
Trinh TT, Santen RAv, Jansen APJ, Meijer EJ (2009) Role of water in silica oligomerization. J Phys Chem C 113:2647–2652
Stanic V, Etsell TH, Pierre AC, Mikula RJ (2001) Chemical kinetics study of the sol-gel processing of GeS2. J Phys Chem A 105:6136–6143
Musat V, Budrugeac P, Gheorghies C (2008) Effect of reagents mixing on the thermal behavior of sol-gel precursors for silica-based nanocomposite thin films. J Therm Anal Calorim 94:373–377
Ahmad S, Deepa M, Agnihotry SA (2008) Effect of salts on the fumed silica-based composite polymer electrolytes. Sol Energy Mater Sol Cells 92:184–189
Felmy AR, Rustad JR, Cho H, Mason MJ (2001) An aqueous thermodynamic model for polymerized silica species to high ionic strength. J Solut Chem 30:509
Schantz Zackrisson A, Martinelli A, Matic A, Bergenholtz J (2006) Concentration effects on irreversible colloid cluster aggregation and gelation of silica dispersions. J Colloid Interface Sci 301:137–144
Trompette JL, Clifton MJ (2004) Influence of ionic specificity on the microstructure and the strength of gelled colloidal silica suspensions. J Colloid Interface Sci 276:475–482
Trompette JL, Meireles M (2003) Ion-specific effect on the gelation kinetics of concentrated colloidal silica suspensions. J Colloid Interface Sci 263:522–527
Burnett SS, Mitchell JW (2013) Dft investigation of the mechanism and chemical kinetics for the gelation of colloidal silica. MRS Proceedings 1547
Marcus Y (2009) Effect of ions on the structure of water: structure making and breaking. Chem Rev 109:1346–1370
Dukhin AS, Goetz PJ (2001) Acoustic and electroacoustic spectroscopy for characterizing concentrated dispersions and emulsions. Adv Colloid Interface Sci 92:73–132
Dukhin AS, Goetz PJ (eds) (2010) Characterisation of liquids, nano- and microparticulates, and porous bodies using ultrasound. Elsevier, Amsterdam
Dukhin AS, Goetz PJ, Thommes M (2010) Seismoelectric effect: a non-isochoric streaming current. 1. Experiment. J Colloid Interface Sci 345:547–553
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Burnett, S.S., Mitchell, J.W. Acoustic and electroacoustic spectroscopic determination of the critical coagulation concentration for the aggregation of fumed silica. J Sol-Gel Sci Technol 74, 661–669 (2015). https://doi.org/10.1007/s10971-015-3645-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-015-3645-0