Abstract
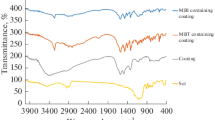
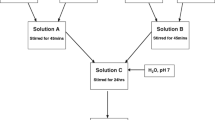
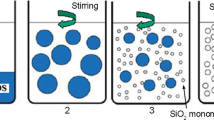
A two-step procedure for synthesizing a new type of hybrid coating is presented. Coatings were synthesized from tetraethyl orthosilicate, 3-(trimethoxysilyl)propyl methacrylate and zirconium(IV) propoxide (ZTP). Zirconium(IV) propoxide was chelated with methacrylic acid. The synthesis, which proceeded at room temperature, was optimized using in situ Fourier transform infrared spectroscopy (FTIR). The effects of ZTP content, ageing and Zr/Si ratio were investigated. The viscosity of the sols increased with the ratio of Zr to Si. The terminal stability of the hybrid coatings was determined by thermogravimetric analysis and their composition and morphology analyzed by FTIR and scanning electron microscopy combined with energy dispersive X-ray spectroscopy. Sols were deposited on the aluminium AA7075-T6 substrate by spin-coating. The corrosion properties in dilute Harrison’s solution of samples coated by hybrid coatings were determined by electrochemical polarization methods. The coatings substantially improved the corrosion resistance of the substrate, as shown by a broad range of passivity extending up to high electrode potentials. The degree of protection was dependent on the content of zirconium and the time of ageing. The strong corrosion protection was ascribed to the bonding between silicon, oxygen and zirconium (Si–O–Si, Si–O–Zr), as proved by the FTIR analysis.












Similar content being viewed by others
References
Davis JR (1999) Corrosion of aluminum and aluminum alloys. ASM International, USA
Chou T, Chandrasekaran C, Cao G (2003) J Sol-Gel Sci Technol 26:321–327
Kendig MW, Buchheit RG (2003) Corrosion 59:379–400
Dimitriev Y, Ivanova Y, Iordanova R (2008) J Univ Chem Technol Metall 43:181–192
Twite RL, Bierwagen GP (1998) Prog Org Coat 33:91–100
Bierwagen G, Brown R, Battocchi D, Hayes S (2010) Prog Org Coat 68:48–61
Restriction of Hazardous Substance Directive (ROHS) 2002/95/EC, www.rohs.eu
Zheng SX, Li JH (2010) J Sol-Gel Sci Technol 54:174–187
Wen J, Wilkes GL (1996) Chem Mater 8:1667–1681
Han Y-H, Taylor A, Mante MD, Knowles KM (2007) J Non-Cryst Solids 353:313–320
Guglielmi M (1997) J Sol-Gel Sci Technol 8:443–449
Metroke TL, Parkhill LR, Knobbe TE (2001) Prog Org Coat 41:233–238
Zheludkevich ML, Salvado IM, Ferreira MGS (2005) J Mater Chem 15:5099–5111
Oubaha M, Etienne P, Calas S, Sempere R, Nedelec JM, Moreau Y (2005) J Non-Cryst Solids 351:2122–2128
Pirhady Tavandashti N, Sanjabi S, Shahrabi T (2009) Prog Org Coat 65:182–186
Du Joshua Y, Damron M, Tang G, Zheng H, Chu C-J, Osborne JH (2001) Prog Org Coat 41:226–232
Voevodin NN, Grebasch NT, Soto WS, Kasten LS, Grant JT, Arnold FE, Donley MS (2001) Prog Org Coat 41:287–293
Rajath Varma PC, Colreavy J, Cassidy J, Oubaha M, McDonagh C, Duffy B (2010) Thin Solid Films 518:5753–5761
Andreatta F, Paussa L, Lanzutti A, Rosero Navaro NC, Aparicio M, Castro Y, Duran A, Ondratschek D, Fedrizzi L (2011) Prog Org Coat 72:3–14
Zheludkevich ML, Serra R, Montemor MF, Miranda Salvado IM, Ferreira MGS (2006) Surf Coat Technol 200:3084–3094
Girardi F, Graziola F, Aldighieri P, Fedrizzi L, Gross S, Di Maggio R (2008) Prog Org Coat 62:376–381
Hosseini SMA, Jafari AH, Jamalizedah E (2009) Electrochim Acta 54:7207–7213
Voevodin NN, Grebasch NT, Soto WS, Arnold FE, Donley MS (2001) Surf Coat Technol 140:24–28
Zheludkevich ML, Serra R, Montemor MF, Yasakau KA, Miranda Salvado IM, Ferreira MGS (2005) Electrochim Acta 51:208–217
Schem M, Schmidt T, Gerwann J, Mittmar M, Veith M, Thompson GE, Molchan IS, Hashimoto T, Skeldon P, Phani AR, Santucci S, Zheludkevich ML (2009) Corrosion Sci 51:2304–2315
Montemor MF, Trabelsi W, Lamaka SV, Yasakau KA, Zheludkevich ML, Bastos AC, Ferreira MGS (2008) Electrochim Acta 53:5913–5922
Gonzales E, Pavez J, Azocar I, Zagal JH, Zhou X, Melo F, Thompson GE, Páez MA (2011) Electrochim Acta 56:7586–7595
Nass R, Schmidt H, Arpac E (1990) SPIE 1328:258–263
Datchary W, Mehner A, Zong HW, Lucca DA, Klopfstein MJ, Ghisleni R, Grimme D, Brinksmeier E (2005) J Sol-Gel Sci Technol 35:245–251
Del Monte F, Cheben P, Grover CP, Mackenzie JD (1999) J Sol-Gel Sci Technol 15:73–85
Oubaha M, Smaili M, Etinne P, Coudray P, Moreau Y (2003) J Non-Cryst Sol 318:305–313
Twite R, Balbyshev S, Bierwagen G (1997) In: Taylor SR, Isaacs HG, Drooman EW (eds) Proceedings of symposium on environmentally acceptable inhibitors and coatings, special publication of the electrochemical society, vol 95–16, p 202
Brinker CJ, Scherer GW (1990) Sol-gel science. The physics and chemistry of sol-gel processing. Academic Press, San Diego, p 358
Pellice SA, Williams RJJ, Sobrados I, Sanz J, Castro Y, Aparicio M, Duran A (2006) J Mater Chem 16:3318–3325
Chmel A, Mazurina EK, Shashkin VS (1990) J Non-Cryst Solids 122:285–290
Chan C-K, Peng S-L, Chu I-M, Ni S-C (2001) Polymer 42:4189–4196
Graziola F, Girardi F, Bauer M, di Maggio R, Rovezzi M, Bertagnolli H, Sada C, Rossetto G, Gross S (2008) Polymer 49:4332–4343
Khaled SM, Sui R, Charpentier PA, Rizkalla AS (2007) Langmuir 23:3988–3995
Rajath Varma PC, Colreavy J, Cassidy J, Oubaha M, Duffy B, McDonagh C (2009) Prog Org Coat 66:406–411
Sorek Y, Zevin M, Reisfeld R, Hurvist T, Ruschin S (1997) Chem Mater 9:670–676
Acknowledgments
The authors thank Barbara Kapun, BSc, for valuable technical help and Prof. Barbara Malič for fruitful discussions regarding the FTIR analysis of solid samples and viscosity measurements. The financial support by the Slovenian Research Agency is acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rodič, P., Iskra, J. & Milošev, I. A hybrid organic–inorganic sol–gel coating for protecting aluminium alloy 7075-T6 against corrosion in Harrison’s solution. J Sol-Gel Sci Technol 70, 90–103 (2014). https://doi.org/10.1007/s10971-014-3278-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-014-3278-8