Abstract
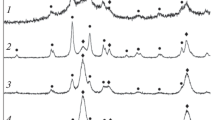
The luminescence lifetime of the green upconversion emission Er3+ ions in ZrO2 nanocrystals was found to be sensitive to the particle size and crystalline phase, as well as to the residual surface contaminants. Erbium doped (0.2 mol% Er2O3) ZrO2 nanocrystals ranging from 54 to 120 nm in size were prepared by a sol–gel process with the presence of nonionic PLURONIC P127 surfactant, and the upconversion emission was characterized. PLURONIC P127 at a molar ratio of 0.0082 promoted both an enhancement in the green upconversion emission as well as a strong reduction of surface contaminants such as CO, CH2, and OH. A fluorescence decay analysis via a simple microscopic rate equation model suggests that crystallite size and nonradiative relaxation mechanisms to different surface contaminants have to be taken into account to explain the observed green luminescence quenching. XRD, FTIR and luminescence lifetime measurements allow the quantification of the nonradiative processes that lead to the green luminescence quenching; and prove the relevance of using nonionic surfactants in the synthesis to reduce residual surface contaminants.







Similar content being viewed by others
References
Liu X, Dong G, Qiao Y, Qiu J (2008) Appl Opt 47(34):6416–6421
Wang G, Peng G, Li P (2011) Acc Chem Res 44(5):322–332
Lüer L, Manzoni C, Egelhaaf HJ, Cerullo G, Oelkrug D, Lanzani G (2006) Physical Review B 73(3):035216
Ghosh P, Patra A (2005) Pramana – J Phys 65(5):901–907
Shaw PE, Ruseckas A, Samuel IDW (2008) Physical Review B 78(24):245201
Kim D, Okahara S, Nakayama M, Shim Y (2008) Physical Review B 78(15):153301
Gamelin DR, Gamelin DR (2001) Top Curr Chem 214:5–23
Zhu Q, Li J-G, Li X, Sun X (2009) Acta Mater 57(20):5975–5985
Chen G, Somesfalean G, Liu Y, Zhang Z, Sun Q, Wang F (2007) Physical Review B 75(19):195204
Lopez-Luke T, De la Rosa E, Salas P, Angeles-Chavez C, Diaz-Torres LA, Bribiesca S (2007) J Phys Chem C 111(45):17110–17117
Angeles-Chavez C, Salas P, Lopez-Luke T, de la Rosa E (2010) Vacuum 84:1226–1231
Solis D, Lopez-Luke T, De la Rosa E, Salas P, Angeles-Chavez C (2009) J Lumin 129:449–455
Salas P, Nava N, Ángeles-Chavez C, De la Rosa E, Díaz-Torres LA (2008) J Nanosci Nanotechnol 8:6431–6436
Meza O, Diaz-Torres LA, Salas P, Rosa EDl, Angeles-Chavez C, Solis D (2009) Journal of Nano Research 5:121–134
Garvie RC, Nicholson PS, Am J (1972) Ceram Soc 55(6):303–305
Hong SJ, Han JI (2007) J Electroceram 18:67–71
De G, Qin W, Zhang J, Zhang J, Wang Y, Cao C, Cui Y (2006) Solid State Commun 137(9):483–487
Nakamura T, Ogawa T, Adachi S, Fujii M (2009) Physical Review B 79(7):075309
de Sousa DF, Nunes LAO (2002) Physical Review B 66(2):024207
Caldiño U, Jaque D, Martín-Rodríguez E, Ramírez MO, García Solé J, Speghini A, Bettinelli M (2008) Physical Review B 77(7):075121
Gómez LA, Maciel GS, Araújo CBd, Patra A (2008) J Appl Phys 103(5):053507
Wolber PK, Hudson BS (1979) Biophys J 28(2):197–210
Rai S, Hazarika S (2008) Opt Mater 30(9):1343–1348
Lewis RM, Torczon V (2002) SIAM J Optim 12(4):1075–1089
Audet C, Dennis JJE (2002) SIAM J Optim 13(3):889–903
Lakowicz JR (2006) Principles of fluorescence spectroscopy, 3rd edn. Springer, Berlin, pp 13–14
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Diaz-Torres, L.A., Salas, P., Angeles-Chavez, C. et al. Green upconversion emission dependence on size and surface residual contaminants in nanocrystalline ZrO2:Er3+ . J Sol-Gel Sci Technol 63, 473–480 (2012). https://doi.org/10.1007/s10971-012-2809-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-012-2809-4