Abstract
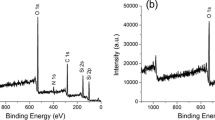
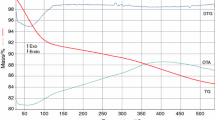
The present contribution reports on our results concerning the synthesis of different binary and ternary oxide systems by using hybrid materials as “composite” precursors. In the last years, we have developed and explored a valuable strategy to yield a very homogeneous dispersion of nanoparticles of early metal transition oxide, MO2 (M = Zr, Hf) inside a silica matrix. This route is based on the use of the sol–gel process to obtain organic–inorganic hybrid silica-based materials embedding the oxide precursors (Zr and/or Hf oxoclusters), which are then calcined at high (T > 500 °C) temperatures to give the desired oxides. The “precursor” hybrid materials are prepared by a modified sol–gel process, involving the copolymerisation of the organically modified oxozirconium or oxohafnium clusters (M4O2(OMc)12 (M = Zr, Hf and OMc = methacrylate) with (methacryloxymethyl)triethoxysilane (MAMTES) or (methacryloxypropyl)trimethoxysilane (MAPTMS). Free radical copolymerisation of the 12 methacrylate groups of the oxoclusters with the methacrylate-functionalised siloxanes allows a stable anchoring of the oxoclusters to the silica network formed by the hydrolysis and condensation of the alkoxy groups. The sol–gel reactions of the two methacrylate-modified silanes methacryloxymethyltriethoxysilane and methacryloxypropyltrimethoxysilane were followed by using two independent time-resolved spectroscopic methods, viz., IR ATR and NMR with the aim to optimise their pre-hydrolysis times and consequently their use as precursors for hybrid materials. As mentioned, thermal treatment at high temperature of the hybrid yields a very homogeneous dispersion of ZrO2 and/or HfO2 nanoparticles in the silica matrix, since the molecular homogeneity of the starting hybrid is retained in the final mixed oxide. This route was successfully applied both to the synthesis of bulk materials and thin films characterised by different compositions (in term of M/Si molar ratios and M nature), heating route (conventional or microwave-assisted) and final temperature of annealing (from RT to 1,100 °C). The first example of the ZrO2–HfO2–SiO2 ternary oxide system was also prepared by this approach. The prepared systems, both in the form of hybrid materials as well as in the final form of binary or ternary oxides, were thoroughly characterised by a wide variety of analytical tools from a compositional, structural, morphological point of view. Moreover, in the case of the binary ZrO2–SiO2 bulk materials, also the evolution under heating was followed by different methods. In particular, the composition of the hybrid as well as of the final oxidic materials was determined by X-Ray Photoelectron Spectroscopy and elemental analysis, whereas FT-IR and multinuclear solid-state NMR spectroscopies shed light on the changes occurring in the composition upon thermal heating and the degree of condensation of the silica network. The morphology and the microstructure of the hybrids and of the oxides were studied by nitrogen sorption and Scanning Electron Microscopy. X-Ray Diffraction, Transmission Electron Microscopy and X-ray Absorption Fine Structure Spectroscopy X-ray Absorption Fine Structure Spectroscopy were used to follow the conversion of the amorphous oxides to the final materials consisting of crystalline zirconia or hafnia dispersed in amorphous silica. On selected systems, functional properties (surface reactivity, dielectric properties) were furthermore investigated. The obtained binary oxides were also used as substrates for functionalisation experiments with (1) dialkycarbamates and (2) long alkyl chains to produce functional materials for catalysis and HPLC applications, respectively.







Similar content being viewed by others
Abbreviations
- AFM:
-
Atomic force microscopy
- ATR:
-
Attenuated total reflection
- BDS:
-
Broadband dielectric spectroscopy
- DRIFT:
-
Diffuse reflectance infrared fourier transform spectroscopy
- EA:
-
Elemental analysis
- EDX:
-
Energy-dispersive X-ray
- FT-IR:
-
Fourier transform infrared spectroscopy
- LA-ICP-MS:
-
Laser ablation inductively coupled plasma mass spectrometry
- NBB:
-
Nano building block
- SEM:
-
Scanning electron microscopy
- SIMS:
-
Secondary ion mass spectrometry
- XAFS:
-
X-ray absorption fine structure spectroscopy
- XPS:
-
X-Ray photoelectron spectroscopy
- XRD:
-
X-Ray diffraction
- TEM:
-
Transmission electron microscopy
- TGA:
-
Thermogravimetric analysis
References
Cox PA (1995) Transition metal oxides: introduction to their electronic structure and properties. Oxford University Press, Oxford
Kung HH (1989) Transition metal oxides: surface chemistry and catalysis. Elsevier, Amsterdam
Rao CNR, Raveau B (2009) Transition metal oxides: structure, properties and synthesis of ceramic oxides. Wiley VCH, Weinheim
Raveau B (2005) J Eur Ceram Soc 25:1965–1969
Fernandez-Garcia M, Martinez-Arias A, Hanson JC, Rodriguez JA (2004) Chem Rev 104:4063–4104
(2007) Special Issue Mater Today 10
Zakrzewska K (2001) Thin Solid Films 391:229–238
Cousin P, Ross RA (1990) Mater Sci Eng A Struct 130:119–125
Lincot D (2010) MRS Bull 35:778–789
Hodes G (2007) Phys Chem Phys 9:181–196
Brinker CJ, Scherer GW (1990) Sol–gel science—the physis and chemistry of sol–gel processing. Academic Press, New York
Frenzer G, Maier WF (2006) Annu Rev Mater Res 36:281–331
Mentruit MP, Palomino GT, Arean CO (2001) Trends Inorg Chem 7:1–14
Bradley DC, Gaur DP, Mehrotra RC (1978) Metal Alkoxides. Academic Press, New York
Mountjoy G, Holland MA, Gunawidjaja P, Wallidge GW, Pickup DM, Newport RJ, Smith ME (2003) J Sol–Gel Sci Technol 26:161, and references therein
Itoh M, Hattori H, Tanabe KJ (1974) J Catal 35:225
Miller JB, Ko EI (1996) J Catal 159:58
Bosman HJM, Kruissink EC, Vanderspoel J, Vandenbrink F (1994) J Catal 148:660
Simhan RG (1983) J Non Cryst Solids 54:335
Feng Z, Postula WS, Erkey C, Philip CV, Akgerman A, Anthony RG (1994) J Catal 148:660
Neumayer DA, Cartier E (2001) J Appl Phys 90:1801
Zhan Z, Zeng HC (1999) J Non Cryst Solids 243:26, and references therein
Miller JB, Ko EI (1997) Catal Today 35:269
Terry KW, Lugmair CG, Tilley TD (1997) J Am Chem Soc 119:9745
Armelao L, Bleiner D, Di Noto V, Gross S, Sada C, Schubert U, Tondello E, Vonmont H, Zattin A (2005) Appl Surf Sci 249:277–294
Armelao L, Eisenmenger-Sittner C, Groenewolt M, Gross S, Sada C, Schubert U, Tondello E, Zattin A (2005) J Mater Chem 15:1838–1848
Armelao L, Bertagnolli H, Gross S, Krishnan V, Lavrencic-Stangar U, Müller K, Orel B, Srinivasan G, Tondello E, Zattin A (2005) J Mater Chem 15:1954–1965
Armelao L, Gross S, Müller K, Pace G, Tondello E, Tsetsgee O, Zattin A Chem Mat 18:6019–6030
Armelao L, Bertagnolli H, Bleiner D, Groenewolt M, Gross S, Krishnan V, Sada C, Schubert U, Tondello E, Zattin A (2007) Adv Funct Mater 17:1671–1681
Mascotto S, Tsetsgee O, Müller K, Maccato C, Smarsly B, Brandhuber D, Tondello E, Gross S (2007) J Mater Chem 17:4387–4399
Bill J, Aldinger F (1995) Adv Mater 7:775–787
Trimmel G, Moraru B, Gross S, Di Noto V, Schubert U (2001) Macromol Symp 175:357
Gross S, Trimmel G, Schubert U, Di Noto V (2002) Polym Adv Technol 13:254
Gross S, Di Noto V, Kickelbick G, Schubert U (2002) Mater Res Soc Symp Proc 726:Q4.1.1
Schubert U, Völkel T, Moszner N (2001) Chem Mater 726:3811
Moraru B, Hüsing N, Kickelbick G, Schubert U, Fratzl P, Peterlik H (2002) Chem Mater 14:2732
Gao Y, Choudhury N, Matisons J, Schubert U (2002) Chem Mater 14:4522
Palacio F, Oliete P, Schubert U, Mijatovic I, Hüsing N, Peterlik H (2004) J Mater Chem 14:1873
Gross S, Di Noto V, Schubert U (2003) J Non Cryst Solids 322:154
Graziola F, Girardi F, Bauer M, Di Maggio R, Rovezzi M, Bertagnolli H, Sada C, Rossetto G, Gross S (2008) Polymer 49:4332
Girardi F, Graziola F, Aldighieri P, Fedrizzi L, Gross S, Di Maggio R (2008) Prog Org Coat 62:376
Sangermano M, Gross S, Pracella L, Priola A, Rizza G (2007) Macromol Chem Phys 208:1730–1736
Di Maggio R, Dirè S, Callone E, Girardi F, Kickelbick G (2008) J Sol Gel Sci Technol 48:188
Di Maggio R, Dirè S, Callone E, Girardi F, Kickelbick G (2010) Polymer 51:832–841
Meneghetti F, Wendel E, Mascotto S, Smarsly BM, Tondello E, Bertagnolli H, Gross S (2010) CrystEngComm 12:1639
Gross S, Zattin A, Di Noto V, Lavina S (2006) Monatsh Chem 137:583–593
Natile MM, Galenda A, Glisenti A, Mascotto S, Gross S (2009) J Non Cryst Solids 355:481–487
Kailasam K, Mascotto S, Gross S, Maccato C, Müller K (2010) J Mater Chem 20:2345–2355
Belli Dell’Amico D, Bertagnolli H, Calderazzo F, D’Arienzo M, Gross S, Rancan M, Scotti R, Smarsly B, Supplit R, Tondello E, Wendel E (2009) Chem Eur J 15:4931–4943
Lavrencic Stangar U, Sassi A, Venzo A, Zattin A, Japelj B, Orel B, Gross S (2009) J Sol Gel Sci Technol 49:329–335
Trimmel G, Gross S, Kickelbick G, Schubert U (2001) Appl Organomet Chem 15:410
Gross S, Kickelbick G, Puchberger M, Schubert U (2003) Monatsh Chem 134:1053
Innocenzi P (2003) J Non Cryst Solids 316:309
Pham QT, Petiand R, Waton H (1991) Proton and carbon NMR spectra of polymers. Penton Press ltd, London
Innocenzi P, Brusatin G, Licoccia S, Di Vona ML, Babonneau F, Alonso B (2003) Chem Mater 15:4790
Schraml J, Chuy ND, Novak P, Chvalovsky V, Mägi M, Lippman E (1978) Collect Czech Chem Commun 43:3202
Jimenez-Morales A, Aranda P, Galvan JC (2003) J Mater Process Technol 5:143–144
Isoda K, Kuroda K, Ogawa M (2000) Chem Mater 12:1702–1707
Luo J, Lannutti J, Seghi R (2001) J Adhes Sci Technol 15:267
Roychen J, Zhang S, Ford WT (1996) Macromolecules 29:1305
Pursch M, Sander LC, Albert K (1996) Anal Chem 68:4107
Kogler FR, Schubert U (2007) Polymer 48:4990
Teo BK (1986) EXAFS: basic principles and data analysis. Springer, Berlin
Koningsberger DC, Prins R (1988) X-ray absorption spectroscopy (principles, applications, techniques of EXAFS, SEXAFS, and XANES). Wiley, New York
Rehr JJ, Albers RC (2002) Rev Mod Phys 72:621
Osendi MI, Moya JS, Serna CJ, Soria J (1985) J Am Ceram Soc 68:135
Garvie RC (1965) J Phys Chem 69:1238
Garvie RC (1978) J Phys Chem 82:218
Nagarajan VS, Rao KJ (1989) J Mater Sci 24:2140
Heuer AH, Claussen N, Kriven WM, Rühle M (1982) J Am Ceram Soc 65:642
Skandan G, Hahn H, Roddy M, Cannon WR (1994) J Am Ceram Soc 77:1706
Scherrer P (1918) Nachr Ges Wiss Göttingen 96
Klug HP, Alexander LE (1974) X-ray diffraction procedures for polycrystalline and amorphous materials. Wiley, New York
Del Monte F, Larsen W, Mackenzie JD (2000) J Am Ceram Soc 83:628
Lange FF (1982) J Mater Sci 17:225
Lange FF (1982) J Mater Sci 17:235
Maria JP, Wickaksana D, Parrette J, Kingon AI (2002) J Mater Res 17:1571
Gmelins L (1973) Handbuch der anorganischen Chemie. Verlag Chemie, Wienheim
Suyama R, Asida T, Kume S (1985) J Am Ceram Soc 68:314
Bykov YV, Rybakov KI, Semenov VE (2001) J Phys D 34:R55
Zhao C, Vleugels J, Groffils C, Luypaert PJ, Van Der Biest O (2000) Acta Mater 48:3795
Clark E, Sutton WH, Lewis DA (1997) Ceram Trans 80:731
Rao KJ, Vaidhyanathan B, Ganguli M, Ramakrishan PA (1999) Chem Mater 11:882
Raner KD, Strauss CR, Vyskoc F, Mokbel L (1993) J Org Chem 58:950
Jhung JH, Lee JH, Forster PM, Ferey G, Cheetham AK, Chang JS (2006) Chem A Eur J 12:7899
Tompsett GA, Conner WC, Yngvesson KS (2006) Chem Phys Chem 7:296
Yao KW, Jaenicke S, Lin JY, Tan KL (1998) Appl Catal B 16:291–301
Acknowledgments
The authors would like firstly to acknowledge all the co-workers (master and PhD students), the colleagues and the cooperation partners (whose names are reported in all the quoted references) who have given, with their work, ideas and knowledge, an outstanding contribution to these studies. The National Research Council (CNR), Italy, the Universities of Padova and Trento, the Italian Consortium INSTM are acknowledged for financial support. The Provincia Autonoma di Trento (PAT, Italy) is gratefully acknowledged for financial support to the PAT project “CeNaCoLi”. The Italian Rectors’ Conference (CRUI), the Ateneo Italo-Tedesco and the Deutsche Akademische Austauschdienst (DAAD) are gratefully acknowledged by both authors for funding of the researchers mobility in the framework of a Vigoni Programme.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to the memory of my husband Klaus Müller.
Professor Klaus Müller, who unexpectedly and prematurely passed away on 1.4.2011, was Full Professor of Chemistry at the University of Trento and a very appreciated and internationally known solid state NMR spectroscopist.
Rights and permissions
About this article
Cite this article
Gross, S., Müller, K. Sol–gel derived silica-based organic–inorganic hybrid materials as “composite precursors” for the synthesis of highly homogeneous nanostructured mixed oxides: an overview. J Sol-Gel Sci Technol 60, 283–298 (2011). https://doi.org/10.1007/s10971-011-2565-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-011-2565-x