Abstract
Gels of the Ti\(_{1-x}\)Ru\(_{x}\)O\(_{2}\) system, where \(x= 0\), 0.001, 0.01, 0.02, 0.05, 0.1, 0.15, 0.2, 0.3 and 0.5 (mol), have been synthesized by a polymeric sol-gel route from Ti (IV)-iso-propoxide and Ru (III) acetyl-acetonate (acac). The mechanisms of the hydrolysis and polycondensation reactions were studied by using Fourier Transform Infrared Spectroscopy (FTIR).
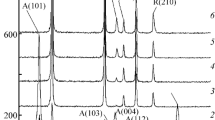
The evolution of the xerogels as a function of temperature was also determined. At temperatures, as low as 200°C, mixtures of antase Ti\(_{1-y}\)Ru\(_y\)O\(_2\) (Ass) solid solution and rutile Ti\(_{1-z}\)Ru\(_{z}\)O\(_{2}\) solid solution (Rss) were attained for compositions with \(x \le\) 0.3. For \(x = 0\), only the anatase phase is present (A) and for \(x = 0.5\), mixtures of anatase Ti\(_{1 -y}\)Ru\(_{y}\)O\(_{2}\) (Ass) solid solution, rutile Ti\(_{1-z}\)Ru\(_{z}\)O\(_{2}\) solid solution (Rss) and Ru\(_{1-a}\)Ti\(_{a}\)O\(_{2}\) (RuO\(_{2}\)ss) solid solution were attained. RuO\(_{2}\) catalyzes the anatase to rutile transformation, even at RuO\(_{2}\) contents as low as 0.001 mol. Although, from 300 to 400°C the solid solubility of RuO\(_{2}\) into rutile-TiO\(_{2}\) phase is located at \(x\le 0.3\), from 500°C that value is located in the 0.05 \(\le x < 0.1\) range. This fact could be due to the metastability of the rutile solid solutions containing ruthenium oxide above 400°C.
According to semiquantitative transmission electron microscopy-energy dispersive X-ray spectroscopy (TEM-EDX) analyses, at 700°C, there are compositional variations in both solid solutions, Rss and RuO\(_{2}\)ss. Thus, the system is chemically heterogeneous. The amount of Ti ions hosted into the RuO\(_{2}\) lattice in the solid solution is lower than that of Ru ions into the rutile-TiO\(_{2}\) lattice. At this temperature, the contents of these solid solutions are \(\approx\!\!17.3\) mol% RuO\(_{2}\) into the TiO\(_{2}\) lattice (the maximum value found) and around 8.0 mol% TiO\(_{2}\) (the maximum value found) into RuO\(_{2}\). The RuO\(_{2}\) volatilization can promote the segregation of the ruthenium oxide giving rise to the heterogeneity and the metastability observed in this system.
Similar content being viewed by others
References
Pârvulescu V, Pârvulescu VI, Popescu G, Julbe C, Guizard C, Cot L (1995) Catal Today 25:385
Guglielmi M, Colombo P, Rigato V, Battaglin G, Boscolo-Boscoletto A, DeBattisti A (1992) J Electrochem Soc 139:1655
Kameyama K, Tsukada K, Yahikozawa K, Takasu Y (1993) J Electrochem Soc 140:966
Mink J, Kristof A, De Battisti A, Daolio S, Nemeth CS (1995) Surf Sci 335:252
Swider KE, Merzbacher CI, Hagans PL, Rolison DR (1997) Chem Mater 9:1248
Panic VV, Dekanski A, Milonjic SK, Atanasoski RT, Nikolic BZ (1999) Colloid Surf A: Physicochem Eng. Aspects 157:269
Zhitomirsky I (1999) J Mater Sci 34:2441
Panic VV, Dekanski A, Wang G, Fedoroff M, Milonjic S, Nikolic B (2003) J Colloid Interf Sci 263:68
Aparicio M, Klein LC (2004) J Sol-Gel Sci Techn 29:81
Málek J, Watanabe A, Mitsuhashi T (2000) J Therm Anal Calorim 60:699
Colomer MT, Jurado JR (2000) Chem Mater 12:923
Gerrard WA, Steele BCH (1978) J Appl Electrochem 8:417
Levedeb VM, Roginskaya YE, Klimasenko NL, Bystrov VI, Venevtsev YN (1976) Zh Neorg Khimii 21:2511
Colomer MT, Jurado JR (1998) J Solid State Chem 141:282
Pizzini S, Buzzanca G, Mari C, Rossi L, Torchio S (1972) Mater Res Bull 7:449
Trasatti S (1991) Electrochim Acta 36:225
Augustynski J, Balsenic L, Hinden J (1978) J Electrochem Soc 125:1093
Lodi G, Asmundis CD, Ardizzone S, Sivieri E, Trasatti S (1981) Surf Technol 14:335
Battisti AD, Lodi G, Cappadonia M, Battaglia G, Kotz R (1989) J Electrochem Soc 136:2596
Wagner W, Kuhnemund L (1989) Cryst Res Technol 24:1009
De Battisti A, Battaglin G, Benedetti A, Kristof J, Liszi J (1995) Chimia 49:17
Vallet CE, Tilak BV, Zuhr RA, Chen CP (1997) J Electrochem Soc 144:1289
Zarzycki J, Prassas M, Phalippou J (1982) J Mater Sci 17:3371
Endo A, Kajitani M, Mukaida M, Shimizu K, Sato GP (1988) Inorg Chim Acta 150:25
Pretch E, Clerc T, Seibl J, Simon W (Eds) (1988) Tablas para la elucidación estructural de compuestos org´anicos por métodos espectroscópicos, vol. I (Alhambra, Madrid) p. 135
Colomer MT, Jurado JR (1997) J Non-Cryst Solids 217:48
Doeuff S, Henry M, Sanchez C, Livage J (1987) J Non-Cryst Solids 89:206
Saito K, Kido H, Nagasawa A (1990) Coord Chem Rev 100:427
Beghi M, Chiurlo P, Costa L, Palladino M, Pirini MF (1992) J Non-Cryst Solids 145:175
Bewick A, Gutiérrez C, Larramona G (1992) J Electroanal Chem 332:155
Chan HYH, Takoudis CG, Weaver MJ (1997) J Catal 172:336
Zhitormirsky I (1998) Mater Lett 33:305
Veseloskaya IE, Spasskaya EK, Sololov VA, Tkachenko VI, Yakimenko LM (1974) Electrokhimiya 10:70
Hine F, Yasuda M, Yoshida T (1977) J Electrochem Soc 124:500
Ito M, Murakami Y, Kaji H, Ohkawauchi H, Yahikozawa K, Takasu Y (1994) J Electrochem Soc 141:1243
Roginskaya YE, Galyamov BS, Belova ID, Shifrina RR, Kozhevnikov VB, Bystrov VI (1982) Soviet Electrochem 18:1179
Hume-Rothery W (1926) J Inst Met 35:295
Bursill LA, Hyde BG (1972) In: Reiss H, McCaldin JO (eds) Progress in solid state chemistry, vol. 7. Pergamon Oxford, p 177
Tagirov VK, Chizhikov DM, Kazenas EK, Shubocchkin LK (1977) J Inorg Chem 20:1133.
Levedeb VM, Roginskaya YE, Klimasenko NL, Bystrov VI, Venevtsev YN (1976) Russ J Inorg Chem 21:1380
Belton DN, Sun YM, White JM (1984) J Phys Chem 88:5172
Daolio S, Facchin B, Pagura C, De Battisti, Barbieri A, Kristóf J (1994) J Mat Chem 4:1255
Colomer MT, Valle FJ, Jurado JR (1997) Eur J Solid State Inorg Chem 34:85
Sheinkman AI, Tymentsev VA, Fotiev AA (1984) Inorg Mater 20:1460
Gouma PI, Mills MJ (2001) J Am Ceram Soc 84:619
Magneli A (1970) In: Eyring L, O'Keefe M (eds) The chemistry of extended defects in non-metallic crystals. North Holland, Amsterdam, p 148
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Colomer, M.T., Velasco, M.J. & Jurado, J.R. Synthesis and thermal evolution of TiO2-RuO2 xerogels. J Sol-Gel Sci Technol 39, 211–222 (2006). https://doi.org/10.1007/s10971-006-8207-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-006-8207-z