Abstract
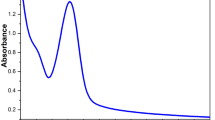
The interplay between the chemical structure of the precursors, internal organization in the end materials and dye retention was investigated for composites (ormosils) doped with rhodamine B. Besides formulations with triethoxysilanes (RTES) only, we synthesized as well organic–inorganic hybrids with addition of titanium isopropoxide (TIP) and maleic anhydride (MA). The organic (R) functionality of RTES was changed from methyl (MeTES), to phenyl (PTES) and octyl (OTES). Atomic force microscopy and electron microscopy, coupled with thermogravimetric analysis prove that hydrophobicity increase stimulates the transition of film structure: from well-defined, compact particles (for MeTES), to a mixture of porous particles and non-granular material (for MeTES/PTES), with extreme results observed for octyl-based composites. For this latter, the apparent homogeneity comes from cluster-like organization, where the primary entities are ‘pseudo—granules’ produced by hydrophobic interactions of oligomeric siloxanes. Controlling the composition and gelation procedure resulted in doped composites with good optical transparency and rhodamine B fluorescence emission bands at around 580 nm. Dye transport inside the inorganic structure is not facilitated when: (a) the particles have a compact (nonporous) inner structure and (b) the recipe does not contain the TIP/MA combination. For silica-based films, the dye is located in the macropores (between the granules) of the material and could be easy removed by washing with acetone. On the contrary, using TIP/MA changes not only the internal composition of the granular-like material (by creating a microporous titania-rich outer-shell of the particles) but also the affinity of the Rh-B to permeate and reside inside these new structures.
Similar content being viewed by others
References
R. Reisfeld, R. Zusman, Y. Cohen, and M. Eyal, Chem. Phys. Lett. 147, 142 (1988).
M. Guglielmi, P. Colombo, L. Mancinelli Degli Esposti, G.C. Righini, S. Pelli, and V. Rigato, J. Non-Cryst. Solids 147, 641 (1992).
M. Canva, G.L. Sanx, P. Georges, A. Brun, F. Chaput, and J.P. Boilot, Opt. Lett. 17, 218 (1992).
F. Bentivegna, M. Canva, P. Georges, A. Brun, F. Chaput, and J.P. Boilot, Appl. Phys. Lett. 62, 1721 (1993).
M. Casalboni, R. Senesi, P. Prosposito, F.D. Matteis, and R. Pizzoferrato, Appl. Phys. Lett. 70, 2969 (1997).
R. Reisfeld, E. Yariv, and H. Minti, Opt. Mater. 8, 31 (1997).
G.H. Hsiue, R.H. Lee, and R.J. Jeng, Chem. Mater 9, 883 (1997).
T. Suratwala, Z. Gardlund, K. Davidson, and R.H. Uhlmann, J. Sol-Gel Sci. Tech. 8, 973 (1997).
J. Lenhart, J.H. Vanzanten, J.P. Dunkers, and R.S. Parnas, Langmuir 16, 8145 (2000).
C.J. Brinker and G.W. Scherer, Sol-Gel Science: The Physics and Chemistry of Sol-Gel Processing (Academic Press, San Diego, 1990).
D. Avnir, S. Braun, and M. Ottolenghi, in Supramolecular Arhitecture: Synthetic Control in Thin Films and Solids, edited by T. Bein, ACS Symp. Ser. 499, 1992, p. 389.
E.J.A. Pope, J. Sol-Gel Sci. Tech. 2, 717 (1994).
N. Wttouck, F. De Schryver, and I. Snykers-Hendrickx, J.Sol-Gel Sci. Tech. 8, 895 (1997).
H. Nakazumi, K. Makita, and R. Nagashiro, J. Sol-Gel Sci. Tech. 8, 901 (1997).
L. Hou, B. Hoffman, M. Mennig, and H. Schmidt, J.Sol-Gel Sci. Tech. 2, 635 (1994).
K. Mongey, J.G. Vos, B.D. Macraith, and C.M. McDonagh, J. Sol-Gel Sci. Tech. 8, 979 (1997).
R. Litran, E. Blanco, M. Ramirez-del-Solar, and L. Esquivias, J. Sol-Gel Sci. Tech. 8, 985 (1997).
J. Garcia, V.M. Castano, M.A. Mondragon, E. Ramirez, F. Gonzalez, A. Campero, and V. Renteria, J. Sol-Gel Sci. Tech. 8, 911 (1997).
T. Schibata, M. Yamane, K. Kamada, K. Ohta, K. Sasaki, and H. Masuhara, J. Sol-Gel Sci. Tech. 8, 959 (1997).
L. Matthews, D. Avnir, A. Modestov, S. Sampath, and O. Lev, J. Sol Gel Sci. Tech. 8, 619 (1997).
X.M. Han, J. Lin, R.B. Xing, J. Fu, and S.B. Wang, Mater. Lett. 57, 1355 (2003).
A.V. Deshpande and U. Kumar, J. Non-Cryst. Solids 306, 149 (2002).
X. Hao, X. Fan, Z. Wang, and M. Wang, Mater. Lett. 51, 245 (2001).
H. Yanagi, T. Hishiki, T. Tobitani, A. Otomo, and S. Mashiko, Chem. Phys. Lett. 292, 332 (1998).
T. Seckin, A. Gultek, and S. Kartaca, Dyes and Pigments 56, 51 (2003).
Y. Yang, M. Wang, G. Qian, Z. Wang, and X. Fan, Opt. Mat. 24, 621 (2004).
K.O. van der Werf, C.A.J. Putman, B.G. de Grooth, F.B. Segerink, E.H. Schipper, N.F. van Hulst, and J. Greve, Rev. Sci. Instrum. 64(10), 2892 (1993).
J.C. Berg in Wettability (Marcel Dekker Inc., New York, 1993), Chap. 2.
C.J. van Oss, R.J. Good, and N.K. Chaudhury, Langmuir 4, 884 (1988).
M. Montes, F.P. Getton, M.S.W. Vong, and P.A. Sermon, J. Sol Gel Sci. Tech. 8, 131 (1997).
R.M. Almeida and E.E. Christensen, J. Sol Gel Sci. Techn. 8, 409 (1997).
K.M.S. Khalil, A.A. Elsamahy and M.S. Elanany, J. Coll. Interf. Sci. 249, 359 (2002).
R. Jones, H.M. Pollock, and D. Geldart, A. Verlinden, Powder Tech. 132(2-3), 196 (2003).
I. Lopez Arbeloa and P. Ruiz Ojeda, Chem. Phys. Lett. 79, 47 (1981).
I. Lopez Arbeloa and K.K. Rohatgi-Mukherjee, Chem. Phys. Lett. 28, 474 (1986).
D. Hinckley, P.G. Seybold, and D.P. Borris, Spectrochim. Acta 42A, 747 (1986).
A. Imhof, M. Megens, J.J. Engelberts, D.T.N. de Lang, R. Sprik, and W.L. Vos, J. Phys. Chem. B 103, 1408 (1999).
R. Tamaki and Y. Chujo, 37-th Int.Symp. Macromol., Gold Coast, Australia, Preprints, 1998, p. 278.
D.L. Ou and A.B. Seddon, J. Non-Cryst. Solids 210, 187 (1997).
D.L. Ou and A.B. Seddon, J. Sol-Gel Sci. Tech. 8, 139 (1997).
W. Que, Z. Sun, Y. Zhou, Y.L. Lam, Y.C. Chan and C.H. Kam, Thin Sol. Films 359, 177 (2000).
L. Frunza, H. Kosslick, U. Bentrup, I. Pitsch, R. Fricke, S. Frunza and A. Schonhals, J. Mol. Struct. 651– 653, 341 (2003).
P. Madhu Kumar, S. Badrinarayanan, and M. Sastry, Thin Sol. Films 358, 122 (2000).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Uricanu, V.I., Donescu, D., Banu, A.G. et al. Influence of Film Structure and Precursor Composition on Rhodamine B Retention in Dye-Dopped Ormosils. J Sol-Gel Sci Technol 34, 23–39 (2005). https://doi.org/10.1007/s10971-005-1259-7
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10971-005-1259-7