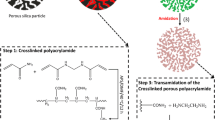
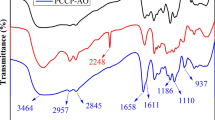
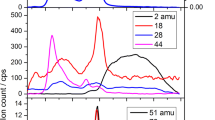
Abstract
A novel polyacrylic acid–ammonium phosphomolybdate (PAA–AMP) adsorbent capable of highly selective adsorption of Cs+ in robust acid solutions was developed. The effects of adsorbent dosage, pH, contact time, temperature, and competing ions on the Cs+ removal performance were investigated. The adsorbent rapid (30 min) selective adsorption of Cs+ in a wide pH (pH = 1–12) range, with little effect of competing ions on the adsorption, and a maximum adsorption capacity of 66.71 mg g−1. The adsorption data fitted well with pseudo-second-order kinetics and the Langmuir isotherm model, and adsorption mechanism is ion exchange between NH4+ and Cs+.
Graphical abstract










Similar content being viewed by others
References
Yang S, Luan Z, Li W, Cheng X, Ye Z, Hu B (2024) Two-dimensional sp2 carbon-conjugated COFs electrode for efficient electro-adsorption of uranium. Sep Purif Technol 330:125378. https://doi.org/10.1016/j.seppur.2023.125378
Kavitha E, Prabhakar S (2022) Review and assessment on the separation of cesium and strontium from the aqueous stream. Desalin Water Treat 251:43–56. https://doi.org/10.5004/dwt.2022.28113
Yang ZC, Guo BX, Hu ZY, Cui JH, Cui JG, Li LN, Hu C, Zhao YB (2023) Highly efficient removal of Cs+ from water by an ionic lamellar carbon nitride framework. J Mater Chem A 11:11859–11865. https://doi.org/10.1039/d3ta00811h
Wang J, Zhuang S (2019) Removal of cesium ions from aqueous solutions using various separation technologies. Rev Environ Sci Bio-Technol 18:231–269. https://doi.org/10.1007/s11157-019-09499-9
Wang J, Jing S, Chen J (2016) Demonstration of a crown ether process for partitioning strontium from high level liquid waste (HLLW). Radiochim Acta 104:107–115. https://doi.org/10.1515/ract-2015-2454
Zhiwu L, Li X, Huang P, Hu H, Li Z, Zhang Q (2019) Mechanochemical activation of antigorite to provide active magnesium for precipitating cesium from the existences of potassium and sodium. Appl Clay Sci 168:223–229. https://doi.org/10.1016/j.clay.2018.11.015
Li W-A, Peng Y-C, Ma W, Huang X-Y, Feng M-L (2022) Rapid and selective removal of Cs+ and Sr2+ ions by two zeolite–type sulfides via ion exchange method. Chem Eng J 442:136377. https://doi.org/10.1016/j.cej.2022.136377
Qin J, Yan L, Han S, Yang X, Guo Y, Li L, Deng T (2023) Tannic acid-assisted prussian blue anchoring on membranes for rapid and recyclable removal of cesium. J Water Process Eng 52:103565. https://doi.org/10.1016/j.jwpe.2023.103565
Gao B, Yu H-R, Zhang H-Y, Liang T, Cheng C-J (2022) High-density immobilization of potassium copper hexacyanoferrate in poly(acrylic acid)/laponite hydrogel for enhanced Cs+ removal. J Environ Chem Eng 10:107979. https://doi.org/10.1016/j.jece.2022.107979
Yang J, Wang M, Zhang L, Lu Y, Di B, Shi K, Hou X (2023) Investigation on the thermal stability of cesium in soil pretreatment and its separation using AMP-PAN resin. J Radioanal Nucl Chem 332:877–885. https://doi.org/10.1007/s10967-023-08775-x
Zhang H, Li CM, Chen XJ, Fu H, Chen YL, Ning SY, Fujita T, Wei YZ, Wang XP (2022) Layered ammonium vanadate nanobelt as efficient adsorbents for removal of Sr2+ and Cs+ from contaminated water. J Colloid Interface Sci 615:110–123. https://doi.org/10.1016/j.jcis.2022.01.164
El-Zahhar AA, Idris AM (2022) Mercury(II) decontamination using a newly synthesized poly(acrylonitrile–acrylic acid)/ammonium molybdophosphate composite exchanger. Toxin Rev 41:25–37. https://doi.org/10.1080/15569543.2020.1824191
Sang H, Mao C, Ming F, Xu L, Wei Y, Wu Y (2022) Selective separation and immobilization process of 137Cs from high-level liquid waste based on silicon-based heteropoly salt and natural minerals. Chem Eng J 449:137842. https://doi.org/10.1016/j.cej.2022.137842
Wang Y, Li F, Mao J, Wang X, Shao Y, Yuan J (2023) Magnetic copper hexacyanoferrate core–shell nanoparticles for effective cesium removal from aqueous solutions. Environ Sci: Water Res Technol 9:1115–1123. https://doi.org/10.1039/D2EW00712F
Chen S, Hu J, Shi J, Wang M, Guo Y, Li M, Duo J, Deng T (2019) Composite hydrogel particles encapsulated ammonium molybdophosphate for efficiently cesium selective removal and enrichment from wastewater. J Hazardous Mater 371:694–704. https://doi.org/10.1016/j.jhazmat.2019.03.047
El-Zahhar AA, Idris AM (2022) Mercury(II) decontamination using a newly synthesized poly(acrylonitrile-acrylic acid)/ammonium molybdophosphate composite exchanger. Toxin Rev 41:25–37. https://doi.org/10.1080/15569543.2020.1824191
Mohammadbagheri Z, Rahmati A, Hoshyarmanesh P (2021) Synthesis of a novel superabsorbent with slow-release urea fertilizer using modified cellulose as a grafting agent and flexible copolymer. Int J Biol Macromol 182:1893–1905. https://doi.org/10.1016/j.ijbiomac.2021.05.191
Baloch H, Usman M, Rizwan S, Hanif A (2019) Properties enhancement of super absorbent polymer (SAP) incorporated self-compacting cement pastes modified by nano silica (NS) addition. Constr Build Mater 203:18–26. https://doi.org/10.1016/j.conbuildmat.2019.01.096
Chen Y, Wang D, Mensaha A, Wang Q, Cai Y, Wei Q (2022) Ultrafast gelation of multifunctional hydrogel/composite based on self-catalytic Fe3+/Tannic acid-cellulose nanofibers. J Colloid Interf Sci 606:1457–1468. https://doi.org/10.1016/j.jcis.2021.08.104
Sethy PK, Mohapatra P, Patra S, Bharatiya D, Swain SK (2021) Antimicrobial and barrier properties of polyacrylic acid/GO hybrid nanocomposites for packaging application. Nano-Struct Nano-Objects 26:100747. https://doi.org/10.1016/j.nanoso.2021.100747
Zhu H, Zhong X (2023) Preparation of ammonium 12-molybdophosphate loaded mesoporous silica for adsorption of cesium ion from aqueous solution. J Radioanal Nucl Chem 332:1901–1907. https://doi.org/10.1007/s10967-023-08860-1
Xian Q, He X, Wang E, Bai Z, Zhao D, Dan H, Ding Y, Zhu W (2021) Preparation of mesoporous MnO2/SBA-15 and its cesium ion adsorption properties. J Radioanal Nucl Chem 327:505–512. https://doi.org/10.1007/s10967-020-07522-w
Dermeche L, Thouvenot R, Hocine S, Rabia C (2009) Preparation and characterization of mixed ammonium salts of Keggin phosphomolybdate. Inorg Chim Acta 362:3896–3900. https://doi.org/10.1016/j.ica.2009.04.049
Zakutevskyy O, Sydorchuk V, Kovtun M, Khalameida S, Skwarek E (2023) Sorption of some cations on ammonium molybdophosphate embedded into structure of silica and titania. Res Chem Intermed 49:2233–2255. https://doi.org/10.1007/s11164-022-04936-x
Liu Q, Ge H, Liu C, Zhang N, Guo Y, Deng T (2023) Highly selective and easily regenerated novel porous polyacrylonitrile-ammonium phosphomolybdate beads for cesium removal from geothermal water. J Water Process Eng 51:103339. https://doi.org/10.1016/j.jwpe.2022.103339
Gao C, He J, Han S, Guo Y, Wang S, Deng T (2023) A highly efficient metal ferrocyanide adsorbent based on zinc phytate for cesium removal. Appl Surf Sci 614:156231. https://doi.org/10.1016/j.apsusc.2022.156231
Awual MR, Yaita T, Kobayashi T, Shiwaku H, Suzuki S (2020) Improving cesium removal to clean-up the contaminated water using modified conjugate material. J Environ Chem Eng 8:103684. https://doi.org/10.1016/j.jece.2020.103684
Agual-Lucas J, Mera-Holguin C, Zambrano-Intriago LA, Ruiz-Reyes E, Gomez-Salcedo Y, Baquerizo-Crespo RJ, Rodriguez-Diaz JM (2023) Kinetics and equilibrium of the adsorption process of dimethoate with corn stalk. Bioremediat J 27:55–65. https://doi.org/10.1080/10889868.2021.1988895
Wang Y, Li K, Fang D, Ye X, Liu H, Tan X, Li Q, Li J, Wu Z (2022) Ammonium molybdophosphate/metal-organic framework composite as an effective adsorbent for capture of Rb+ and Cs+ from aqueous solution. J Solid State Chem 306:122767. https://doi.org/10.1016/j.jssc.2021.122767
Ma L, Huang Y, Zhao K, Deng H, Qiang T, Yan M (2021) Removal of uranium from acidic aqueous solution by natural fluorapatite. J Environ Chem Eng 9:106600. https://doi.org/10.1016/j.jece.2021.106600
Mazrouaa A, Mansour N, Youssif M, Shenashen M, Awual M (2019) Nano–composite multi-wall carbon nanotubes using poly (p-phenylene terephthalamide) for enhanced electric conductivity. J Environ Chem Eng 7:103002. https://doi.org/10.1016/j.jece.2019.103002
Largitte L, Pasquier R (2016) A review of the kinetics adsorption models and their application to the adsorption of lead by an activated carbon. Chem Eng Res Des 109:495–504. https://doi.org/10.1016/j.cherd.2016.02.006
Wang J, Guo X (2020) Adsorption kinetic models: physical meanings, applications, and solving methods. J Hazardous Mater 390:122156. https://doi.org/10.1016/j.jhazmat.2020.122156
Mohammadi S, Faghihian H (2019) Elimination of Cs+ from aquatic systems by an adsorbent prepared by immobilization of potassium copper hexacyanoferrate on the SBA-15 surface: kinetic, thermodynamic, and isotherm studies. Environ Sci Pollut Res 26:12055–12070. https://doi.org/10.1007/s11356-019-04623-2
Pérez-Marín AB, Zapata VM, Ortuño JF, Aguilar M, Sáez J, Lloréns M (2007) Removal of cadmium from aqueous solutions by adsorption onto orange waste. J Hazardous Mater 139:122–131. https://doi.org/10.1016/j.jhazmat.2006.06.008
Loukidou MX, Zouboulis AI, Karapantsios TD, Matis KA (2004) Equilibrium and kinetic modeling of chromium(VI) biosorption by Aeromonas caviae. Colloids Surf A: Physicochem Eng Aspects 242:93–104. https://doi.org/10.1016/j.colsurfa.2004.03.030
Ho YS, McKay G (2000) The kinetics of sorption of divalent metal ions onto sphagnum moss peat. Water Res 34:735–742. https://doi.org/10.1016/S0043-1354(99)00232-8
Tang J-H, Jin J-C, Li W-A, Zeng X, Ma W, Li J-L, Lv T-T, Peng Y-C, Feng M-L, Huang X-Y (2022) Highly selective cesium(I) capture under acidic conditions by a layered sulfide. Nat Commun 13:658. https://doi.org/10.1038/s41467-022-28217-8
Liu S, Zu J, Han G, Pan X, Xue Y, Diao J, Tang Q, Jin M (2024) Ammonium phosphomolybdate-modified UiO-66 as an efficient adsorbent for the selective removal of 137Cs from radioactive wastewater. Sep Purif Technol 329:125073. https://doi.org/10.1016/j.seppur.2023.125073
Sun X, Wang Y, Zhang H, Shen Y, Shi Q, Chen H, Sun J, Zhang Z (2022) Fabrication and performance of the ammonium molybdophosphate/polysulfone mixed matrix membranes for rubidium adsorption in aqueous solution. J Appl Polym Sci 139:51798. https://doi.org/10.1002/app.51798
Zhou X, Zhou X (2014) The unit problem in the thermodynamic calculation of adsorption using the Langmuir equation. Chem Eng Commun 201:1459–1467. https://doi.org/10.1080/00986445.2013.818541
Polley K, Bera J (2022) Adsorptive removal of methyl blue dye through magnetically retrievable BaFe12O19-activated charcoal-chitosan composite powder: kinetics, isotherms and thermodynamics studies. Int J Environ Anal Chem. https://doi.org/10.1080/03067319.2022.2128790
Olatunji MA, Khandaker MU, Mahmud HNME, Amin YM (2015) Influence of adsorption parameters on cesium uptake from aqueous solutions- a brief review. RSC Adv 5:71658–71683. https://doi.org/10.1039/C5RA10598F
Khandaker S, Chowdhury MF, Awual MR, Islam A, Kuba T (2021) Efficient cesium encapsulation from contaminated water by cellulosic biomass based activated wood charcoal. Chemosphere 262:127801. https://doi.org/10.1016/j.chemosphere.2020.127801
Wei H, Xi Q, Xa C, Daying G, Ding F, Yang Z, Wang S, Li J, Huang S (2018) Molybdenum carbide nanoparticles coated into the graphene wrapping N-doped porous carbon microspheres for highly efficient electrocatalytic hydrogen evolution both in acidic and alkaline media. Adv Sci 5:1700733. https://doi.org/10.1002/advs.201700733
Tan Z, Dong L, Huang Z, Du L, Wang X (2016) A theoretical study on the selective adsorption of NH4+ and Cs+ on the phosphomolybdate ion. Colloids Surf A: Physicochem Eng Aspects 502:74–80. https://doi.org/10.1016/j.colsurfa.2016.05.019
Wu Y, Zhang X-X, Wei Y-Z, Mimura H (2017) Development of adsorption and solidification process for decontamination of Cs-contaminated radioactive water in Fukushima through silica–based AMP hybrid adsorbent. Sep Purif Technol 181:76–84. https://doi.org/10.1016/j.seppur.2017.03.019
Mhatre A, Agarwal C, Kumar S, Patra S, Tripathi R (2022) Selective and fast separation of cesium ions by in situ synthesized ammonium molybdophosphate-like moieties in a polymer gel. ACS Appl Polym Mater 4:7564–7574. https://doi.org/10.1021/acsapm.2c01240
Saha S, Singhal R, Basu H, Pimple M (2016) Ammonium molybdate phosphate functionalized silicon dioxide impregnated in calcium alginate for highly efficient removal of 137Cs from aquatic bodies. RSC Adv 6:95620–95627. https://doi.org/10.1039/C6RA20283G
Sun C, Zhang F, Wang X, Cheng F (2015) Facile preparation of ammonium Molybdophosphate/Al-MCM-41 composite material from natural clay and its use in cesium ion adsorption. Eur J Inorg Chem 2015:2125–2131. https://doi.org/10.1002/ejic.201500114
Park Y, Shin WS, Choi S-J (2013) Ammonium salt of heteropoly acid immobilized on mesoporous silica (SBA-15): an efficient ion exchanger for cesium ion. Chem Eng J 220:204–213. https://doi.org/10.1016/j.cej.2013.01.027
Fu C, Tan Z, Cheng J, Xie J, Dai X, Du Y, Zhu S, Wang S, Yan M (2023) Effective removal of cesium by ammonium molybdophosphate–polyethylene glycol magnetic nanoparticles. J Environ Chem Eng 11:110544. https://doi.org/10.1016/j.jece.2023.110544
Funding
This research was Supported by the Natural Science Foundation of Sichuan Province, China (No. 2022NSFSC1228, 2022JDTD0017); and CAEA Innovation Center for Geological Disposal of High-Level Radioactive Waste (No. CXJJ21102209); and Doctoral Foundation of Southwest University of Science and Technology (Grant No. 23zx7111).
Author information
Authors and Affiliations
Contributions
The manuscript was written through contributions of all authors. CF: Conceptualization, Investigation, Formal analysis, Methodology, Writing—original draft. XW: Writing—review & editing, Validation. JL: Formal analysis, Validation. JC: Investigation, Formal analysis. SZ: Supervision, Writing—review & editing. MY: Supervision, Resources, Conceptualization, Project administration.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fu, C., Wei, X., Lian, J. et al. A facile synthesis of polyacrylic acid–ammonium phosphomolybdate microspheres for the highly selective removal of cesium. J Radioanal Nucl Chem 333, 2207–2220 (2024). https://doi.org/10.1007/s10967-024-09416-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-024-09416-7