Abstract
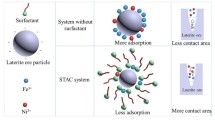
For the leaching process of uranium ore, the leaching rate, leaching efficiency, and energy consumption are hot research topics. In order to meet the industry’s demand for natural uranium and ensure the stable supply of uranium products, this article proposes the use of surfactants to improve the leaching efficiency and leaching rate of uranium ore, thereby achieving better leaching results at larger particle sizes and reducing grinding energy consumption. This article focuses on the core samples of a certain mine and adopts a stirring leaching method. Firstly, the acid stirring leaching process conditions were optimized. Based on this, the types and concentrations of surfactants were selected, and the influence of the selected surfactants on the stirring leaching law of the ore was investigated. The results indicate that the addition of cetyltrimethylammonium bromide (CTAB) cationic surfactant has the highest leaching efficiency in the optimal conventional stirring leaching environment for ores. Compared with the stirring leaching effect without CTAB, after adding 50 mg/L CTAB for leaching, the leaching rate of uranium ore increased from 90.79 to 96.34% within the same leaching cycle; By adding 50 mg/L CTAB to leach ore with a particle size of − 60 mesh, the uranium leaching rate increased from 35.09 to 87.79%, achieving effective leaching of uranium ore with coarser particles; Through analysis such as BET and SEM, it was determined that the addition of CTAB promoted the solution to enter the microporous gaps between ores, achieving an increase in leaching rate. Surfactants reduced the thickness of the liquid film formed on the ore surface and the diffusion time of the liquid film, which was the reason for the improvement of uranium leaching effect.











Similar content being viewed by others
References
Qiang W (2009) China needing a cautious approach to nuclear power strategy[J]. Energy Policy 37(7):2487
Jiang ZY, Pan ZQ, Xing J, Yu F (2015) Research on the life cycle greenhouse gas emissions of China’s nuclear power energy Chain [J]. China Environ Sci 35(11):3502
Chen ZS (2004) The application of some advanced technologies in the uranium mining industry [J]. World Nucl Geosci 02:87
Wang YQ, Xue YS, Duan HJ (2016) Optimization of acid stirring leaching process for a certain uranium mine [J]. Uranium Min Metallurg 35(03):182
Hill HJ, Reisberg J, Stegemeier GL (1973) Aqueous surfactant systems for oil recovery[J]. J Petrol Technol 25(2):186
Van Santvoort J, Golombok M (2015) Viscoelastic surfactants for diversion control in oil recovery[J]. J Petrol Sci Eng 135:671
Deng X, Kamal MS, Patil S, Hussain SMS, Zhou X (2019) A review on wettability alteration in carbonate rocks: wettability modifiers[J]. Energy Fuels 34(1):31
Kamal MS, Hussein IA, Sultan AS (2017) Review on surfactant flooding: phase behavior, retention, IFT, and field applications[J]. Energy Fuels 31(8):7701
Olajire AA (2014) Review of ASP EOR (alkaline surfactant polymer en-hanced oil recovery) technology in the petroleum industry: prospects and challenges[J]. Energy 77:963
Alarifi SA, Mahmoud MA, Shahzad KM (2018) Interactions of DTPA chelating agent with sandstone rocks during EOR: rock surface charge study[J]. Fuel 232:684
Pal S, Mushtaq M, Banat F, Ali SA (2018) Review of surfac-tant-assisted chemical enhanced oil recovery for carbonate reservoirs: challenges and future perspectives[J]. Pet Sci 15:77
Wu AX, Ai CM, Wang YM, Hu KJ (2014) The effect of surfactants on the permeability of copper ore heap leaching [J]. J Cent South Univ: Nat Sci Ed 45(3):895
Cai YY, Ma LW, Xi XL, Nie ZH, Nie ZL (2020) Separation of tungsten and molybdenum using selective precipitation with manganese sulfate assisted by cetyltrimethyl ammonium bromide (CTAB)[J]. Hydrometal-lurgy 198:105494
Lv Y, Lv JW, Zhou JL, Shen J (2016) Study on surfactant improving leaching rate of uranium ore [J]. Rare Met 40(2):182
Wang DY, Kan JQ (1982) Uranium extraction and refinement technology [M]. At Energy Press, Beijing, pp 67–115
Xu WD, Sun R, Zhu XP, Liu PH (2010) Improvement of the method for determining trace uranium in ores using ammonium vanadate micro ti-tration with titanium reduction [J]. Rock Miner Test 29(03):325
Gou YC, Feng L, Zhang Y, Ruan JZ, Song LL (2011) The effect of par-ticle size on the determination of wettability by the Washburn method [J]. Lab Res Explor 30(12):17
Ren BY, Wang SL, Sun L (2005) Determination of contact angle of organic liquids on powders [J]. Liaoning Chem Ind 34(5):219–223
Zeng X (2006) Research on surface properties of filter media: determination of wetting contact angle [J]. J Northwest Univ Natl 27(63):25–27
Shen DM, Yu CR, Xiao JQ (2009) Research and exploration of wetting properties of several conventional filter media [J]. Ind Saf Environ Prot 35(5):8–10
Wei H, Xu CT, Han QP et al (2005) Rapid determination of surface free enthalpy change of sediment in the Yangtze River Estuary using powder contact angle method [J]. Water Purif Technol 24(6):1–3
Luan H, Jiao NL (2010) Exploration of measuring the wettability (contact angle) of conventional filter media using pressure method [J]. J Lanzhou Jiaotong Univ 29(3):139–141
Huo YB (2015) A uranium mine gravity flotation enrichment concentrate acid stirring leaching worker art research [J]. Uranium Min Metallurg 34(2):122–126
García GG, Coello-Velázquez AL, Pérez BF, Menéndez-Aguado JM (2021) Variability of the ball mill bond’s standard test in a Ta Ore due to the lack of standardization. Metals 11(10):1606
Xi YF, Frost RL, He HP, Kloprogge T, Bostrom T (2005) Modification of Wyoming montmorillonite surfaces using a cationic surfactant[J]. Langmuir 21(19):8675
Acknowledgements
This project was mainly supported by the Nuclear Energy Development Project (technology for the mining and metallurgy of associated uranium resources—on the demonstration of uranium co-mining in Bayan Ura, Inner Mongolia ) , National Natural Science Foundation of China (21761002), China Uranium Industry Co., Ltd.—the Foundation of State Key Laboratory of Nuclear Resources and Environment Joint Innovation Fund Project (2022NRE-LH-15) and Key Project of Jiangxi Natural Science Foundation (20232ACB203014).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Feng, J., Guo, J., Tang, R. et al. The effect of surfactants on the efficiency and consumption reduction of acid stirred leaching of uranium ore. J Radioanal Nucl Chem 333, 1873–1881 (2024). https://doi.org/10.1007/s10967-024-09405-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-024-09405-w