Abstract
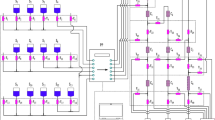
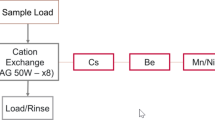
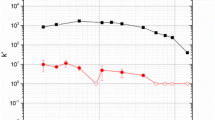
A rapid method to separate Ta, Po, Au, Pt, and W from a sample containing mixed fission and activation products was developed using three commercially available extraction chromatography resins stacked in series. When the separation was tested using all the target activation products, even those with short half-lives were measurable. Combining multiple extraction chromatography resin cartridges in tandem allows for multiple short-lived activation products to be separated from one sample addition without the complication of multiple steps.

Similar content being viewed by others
References
Gonzales ER et al (2005) Evaluation of mass spectrometry and radiation detection forthe analysis of radionuclides. J Radioanal Nucl Chem 263(2):457–465
Hanson S, Oldham W (2021) Weapons radiochemistry: trinity and beyond. Nucl Technol 207:S295–S308
Nichols AL, Verpelli M (2007) Handbook of nuclear data for safeguards. In: 2007: International atomic energy agency (IAEA), p 94
Schwantes JM, Orton CR, Clark RA (2012) Analysis of a nuclear accident: fission and activation product releases from the Fukushima Daiichi nuclear facility as remote indicators of source identification, extent of release, and state of damaged spent nuclear fuel. Environ Sci Technol 46(16):8621–8627
Shozugawa K, Nogawa N, Matsuo M (2012) Deposition of fission and activation products after the Fukushima Dai-ichi nuclear power plant accident. Environ Pollut 163:243–247
Dry DE, Bauer E, Petersen LA (2005) Rapid separation of fresh fission products. J Radioanal Nucl Chem 263(1):19–22
Seiner BN et al (2016) Fission product analysis of HEU irradiated within a boron carbide capsule: comparison of detection methodology at PNNL and AWE. J Radioanal Nucl Chem 307(3):1729–1734
Wren MS et al (2023) Chemical separation and measurement of platinum activation products. Talanta 260:124587
Bennett KT et al (2019) Rapid activation product separations from fission products and soil matrixes. J Radioanal Nucl Chem 322(2):281–289
Faris JP (1978) Separation of metal ions by anion exchange in mixtures of hydrochloric acid and hydrofluoric acid. United States
Pourmand A, Dauphas N (2010) Distribution coefficients of 60 elements on TODGA resin: application to Ca, Lu, Hf, U and Th isotope geochemistry. Talanta 81(3):741–753
Emergy JF, Leddicotte GW (1961) The radiochemistry of gold. National academy of sciences nuclear science series, p 1–40
Leddicotte GW (1961) The radiochemistry of platinum. National academy of sciences nuclear science series, p 1–30
Leddicotte GW, Mullins WT (1961) The radiochemistry of tungsten. National academy of sciences nuclear science series, p 1–40
Figgins PE (1961) The radiochemistry of polonium. National academy of sciences nuclear science series, p 1–74
Steinberg EP (1961) The Radiochemistry of Niobuim and Tantalum, in National Academy of Sciences Nuclear Science Series, p 1–57
Douglas M et al (2009) Separation and quantification of chemically diverse analytes in neutron irradiated fissile materials. J Radioanal Nucl Chem 282(1):63
Morrison SS et al (2017) Activation product analysis in a mixed sample containing both fission and neutron activation products. J Radioanal Nucl Chem 314(3):2501–2506
Morrison SS et al (2019) Isolation of Au and Pt radionuclides from deuteron-irradiated platinum using TBP resin. J Radioanal Nucl Chem 319(3):679–686
Morrison SS et al (2016) A chemical separation procedure using ionic liquid extraction for 59Fe and 55Fe quantification. J Radioanal Nucl Chem 307(3):2479–2485
Snow M, Ward J (2020) Fundamental distribution coefficient data and separations using eichrom extraction chromatographic resins. J Chromatogr A 1620:460833
Snow MS et al (2017) Extraction chromatographic separations of tantalum and tungsten from hafnium and complex matrix constituents. J Chromatogr A 1484:1–6
Snow M, Ward J (2020) Fundamental distribution coefficient data and separations using eichrom extraction chromatographic resins. J Chromatogr A 1620:460833
Lemons B et al (2018) A rapid method for the sequential separation of polonium, plutonium, americium and uranium in drinking water. Appl Radiat Isot 136:10–17
Horwitz PE, Chiarizia R, Dietz ML (1992) A novel strontium-selective extraction chromatographic resin. Solvent Extr Ion Exch 10(2):313–336
Agrawal YK (2002) Liquid–liquid extraction, separation recovery and transport of tantalum by crown-ether. Talanta 58(5):875–882
Caletka R, Hausbeck R, Krivan V (1986) The distribution of elements between polyether-type polyurethane foam, cyclic polyethers and hydrofluoric acid solution. Talanta 33(3):219–224
Camagong CT, Honjo T (2002) Separation of gold(III) as Its Ion-pair complex with 18-crown-6 from hydrochloric acid media by means of solvent extraction. Anal Sci/Suppl 17icas:i725–i728
Despotopulos JD, Kmak KN (2021) Rapid isolation of 197m, gHg from proton irradiated Au foils. J Radioanal Nucl Chem 327(3):1349–1354
Koshima H, Onishi H (1985) Extraction of Iron(III) with crown ethers from hydrochloric acid solutions. Anal Sci 1:389–390
Koshima H, Onishi H (1987) Adsorption of iron(III), gold(III), gallium(III), thallium(III) and antimony(V) on crown ether polymer from hydrochloric acid solution. Anal Sci 3(5):417–421
Hubert-Pfalzgraf LG, Tsunoda M (1980) Synthesis and characterisation of niobium and tantalum crown ether complexes. Inorg Chim Acta 38:43–48
Zhang N, Kou J, Sun C (2023) Investigation on gold-ligand interaction for complexes from gold leaching: a DFT study. Molecules 28:1508. https://doi.org/10.3390/molecules28031508
Grahek Ž, Mačefat MR (2004) Isolation of iron and strontium from liquid samples and determination of 55Fe and 89,90Sr in liquid radioactive waste. Anal Chim Acta 511(2):339–348
Acknowledgements
This work was sponsored by the Office of the Deputy Assistant Secretary of Defense for Nuclear Matters and by the U.S. Department of Energy’s National Nuclear Security Administration, Office of Defense Nuclear Nonproliferation Research and Development. We would like to thank the Radiological Processing Laboratory (RPL) service center at PNNL for gamma emission analysis data. PNNL is operated by Battelle for the U.S. Department of Energy (DOE) under Contract No. DE-AC05-76RL0-1830.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing or conflicting interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Herman, S., Warzecha, E., Stene, R. et al. Evaluation of SR/ TEVA/ TRU triple stack for separation of activation products. J Radioanal Nucl Chem 333, 2351–2360 (2024). https://doi.org/10.1007/s10967-024-09379-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-024-09379-9