Abstract
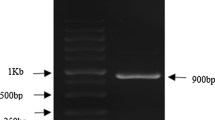
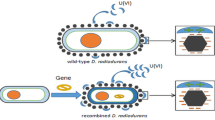
Deinococcus radiodurans (DR) exhibits strong resistance to ionizing radiation. In this study, by constructing a radiation-resistant genetically engineered strain overexpressing the Cs gene, the tolerance of the bacterium to aluminum ions was enhanced, thereby achieving the goal of microbial sustainable remediation of uranium-contaminated environments. Methods: 1. Extraction of the recombinant plasmid pRADK-Cs, transformation into DR, and verification. 2. Investigation of factors such as time and initial uranium concentration on the efficiency of uranium accumulation by the recombinant strain, characterized by changes in functional groups and surface morphology before and after accumulation using techniques such as scanning electron microscope. Conclusions: The recombinant strain Deino-Cs can reduce the inhibitory effect of aluminum ions on uranium accumulation capability, and it exhibits a higher uranium accumulation rate compared to the wild-type strain.



























Similar content being viewed by others
References
Haas R, Mez L, Ajanovic A (eds) (2019) The technological and economic future of nuclear power. Springer Fachmedien Wiesbaden. https://doi.org/10.1007/978-3-658-25987-7
Xing W, Wang A, Yan Q, Chen S (2017) A study of China’s uranium resources security issues: Based on analysis of China’s nuclear power development trend. Ann Nucl Energy 110:1156–1164. https://doi.org/10.1016/j.anucene.2017.08.019
Yin M, Tsang DCW, Sun J et al (2020) Critical insight and indication on particle size effects towards uranium release from uranium mill tailings: geochemical and mineralogical aspects. Chemosphere 250:126315. https://doi.org/10.1016/j.chemosphere.2020.126315
Mkandawire M (2013) Biogeochemical behaviour and bioremediation of uranium in waters of abandoned mines. Environ Sci Pollut Res 20(11):7740–7767. https://doi.org/10.1007/s11356-013-1486-3
Zhang S, Chen L, Qu Z et al (2023) Confining Ti-oxo clusters in covalent organic framework micropores for photocatalytic reduction of the dominant uranium species in seawater. Chem 9(11):3172–3184. https://doi.org/10.1016/j.chempr.2023.06.008
Felipe-Sotelo M, Hinchliff J, Field LP, Milodowski AE, Preedy O, Read D (2017) Retardation of uranium and thorium by a cementitious backfill developed for radioactive waste disposal. Chemosphere 179:127–138. https://doi.org/10.1016/j.chemosphere.2017.03.109
Zhang H, Liu W, Li A et al (2019) Three mechanisms in one material: uranium capture by a polyoxometalate-organic framework through combined complexation, chemical reduction, and photocatalytic reduction. Angew Chem Int Ed 58(45):16110–16114. https://doi.org/10.1002/anie.201909718
Moraes MLBD, Ladeira ACQ (2021) The role of iron in the rare earth elements and uranium scavenging by Fe–Al-precipitates in acid mine drainage. Chemosphere 277:130131. https://doi.org/10.1016/j.chemosphere.2021.130131
Shen J, Schäfer A (2014) Removal of fluoride and uranium by nanofiltration and reverse osmosis: a review. Chemosphere 117:679–691. https://doi.org/10.1016/j.chemosphere.2014.09.090
Zhang H, Li A, Li K et al (2023) Ultrafiltration separation of Am(VI)-polyoxometalate from lanthanides. Nature 616(7957):482–487. https://doi.org/10.1038/s41586-023-05840-z
Singh A, Catalano JG, Ulrich KU, Giammar DE (2012) Molecular-scale structure of uranium(VI) immobilized with goethite and phosphate. Environ Sci Technol 46(12):6594–6603. https://doi.org/10.1021/es300494x
Mikutta C, Langner P, Bargar JR, Kretzschmar R (2016) Tetra- and hexavalent uranium forms bidentate-mononuclear complexes with particulate organic matter in a naturally uranium-enriched Peatland. Environ Sci Technol 50(19):10465–10475. https://doi.org/10.1021/acs.est.6b03688
Wen H, Pan Z, Giammar D, Li L (2018) Enhanced uranium immobilization by phosphate amendment under variable geochemical and flow conditions: insights from reactive transport modeling. Environ Sci Technol 52(10):5841–5850. https://doi.org/10.1021/acs.est.7b05662
Thakare M, Sarma H, Datar S et al (2021) Understanding the holistic approach to plant-microbe remediation technologies for removing heavy metals and radionuclides from soil. Curr Res Biotechnol 3:84–98. https://doi.org/10.1016/j.crbiot.2021.02.004
Gawrońska H, Bakera B (2015) Phytoremediation of particulate matter from indoor air by Chlorophytum comosum L. plants. Air Qual Atmos Health 8(3):265–272. https://doi.org/10.1007/s11869-014-0285-4
Akinbile CO, Ogunrinde TA, Che Bt Man H, Aziz HA (2016) Phytoremediation of domestic wastewaters in free water surface constructed wetlands using Azolla pinnata. Int J Phytoremediat 18(1):54–61. https://doi.org/10.1080/15226514.2015.1058330
Rizwan M, Ali S, Rizvi H et al (2016) Phytomanagement of heavy metals in contaminated soils using sunflower: A review. Crit Rev Environ Sci Technol 46(18):1498–1528. https://doi.org/10.1080/10643389.2016.1248199
Sánchez-Castro I, Martínez-Rodríguez P, Jroundi F, Solari PL, Descostes M, Merroun ML (2020) High-efficient microbial immobilization of solved U(VI) by the stenotrophomonas strain Br 8. Water Res 183:116110. https://doi.org/10.1016/j.watres.2020.116110
Song J, Han B, Song H et al (2019) Nonreductive biomineralization of uranium by Bacillus subtilis ATCC–6633 under aerobic conditions. J Environ Radioact 208–209:106027. https://doi.org/10.1016/j.jenvrad.2019.106027
Brookshaw DR, Pattrick RAD, Lloyd JR, Vaughan DJ (2012) Microbial effects on mineral–radionuclide interactions and radionuclide solid-phase capture processes. Mineral Mag 76(3):777–806. https://doi.org/10.1180/minmag.2012.076.3.25
Kazy SK, D’Souza SF, Sar P (2009) Uranium and thorium sequestration by a Pseudomonas sp.: mechanism and chemical characterization. J Hazard Mater 163(1):65–72. https://doi.org/10.1016/j.jhazmat.2008.06.076
Lopez-Fernandez M, Romero-González M, Günther A, Solari PL, Merroun ML (2018) Effect of U(VI) aqueous speciation on the binding of uranium by the cell surface of Rhodotorula mucilaginosa, a natural yeast isolate from bentonites. Chemosphere 199:351–360. https://doi.org/10.1016/j.chemosphere.2018.02.055
Macaskie LE, Bonthrone KM, Yong P, Goddard DT (2000) Enzymically mediated bioprecipitation of uranium by a Citrobacter sp.: a concerted role for exocellular lipopolysaccharide and associated phosphatase in biomineral formation. Microbiology. 146(8):1855–1867. https://doi.org/10.1099/00221287-146-8-1855
Chen Z, Wang S, Hou H et al (2022) China’s progress in radionuclide migration study over the past decade (2010–2021): sorption, transport and radioactive colloid. Chin Chem Lett 33(7):3405–3412. https://doi.org/10.1016/j.cclet.2022.02.054
Williamson AJ, Binet M, Sergeant C (2023) Radionuclide biogeochemistry: from bioremediation toward the treatment of aqueous radioactive effluents. Crit Rev Biotechnol. https://doi.org/10.1080/07388551.2023.2194505
Basu B (2022) The radiophiles of Deinococcaceae family: resourceful microbes for innovative biotechnological applications. Curr Res Microb Sci 3:100153. https://doi.org/10.1016/j.crmicr.2022.100153
Righi H, Arruda-Neto JDT, Gomez JGC, Da Silva LF, Somessari ESR, Lemos ACC (2020) Exposure of Deinococcus radiodurans to both static magnetic fields and gamma radiation: observation of cell recuperation effects. J Biol Phys 46(3):309–324. https://doi.org/10.1007/s10867-020-09554-5
Kumar J, Ghosh P, Kumar A (2021) Ultraviolet-B radiation stress-induced toxicity and alterations in proteome of Deinococcus radiodurans. Microb Physiol 31(1):1–15. https://doi.org/10.1159/000512018
Barone P, Rosellini D, LaFayette P, Bouton J, Veronesi F, Parrott W (2008) Bacterial citrate synthase expression and soil aluminum tolerance in transgenic alfalfa. Plant Cell Rep 27(5):893–901. https://doi.org/10.1007/s00299-008-0517-x
Hapeta P, Rakicka-Pustułka M, Juszczyk P, Robak M, Rymowicz W, Lazar Z (2020) Overexpression of citrate synthase increases isocitric acid biosynthesis in the yeast Yarrowia lipolytica. Sustainability 12(18):7364. https://doi.org/10.3390/su12187364
Chauhan DK, Yadav V, Vaculík M et al (2021) Aluminum toxicity and aluminum stress-induced physiological tolerance responses in higher plants. Crit Rev Biotechnol 41(5):715–730. https://doi.org/10.1080/07388551.2021.1874282
Niu H, Leng Y, Ran S et al (2020) Toxicity of soil labile aluminum fractions and aluminum species in soil water extracts on the rhizosphere bacterial community of tall fescue. Ecotoxicol Environ Saf 187:109828. https://doi.org/10.1016/j.ecoenv.2019.109828
Chen ZC, Liao H (2016) Organic acid anions: an effective defensive weapon for plants against aluminum toxicity and phosphorus deficiency in acidic soils. J Genet Genomics 43(11):631–638. https://doi.org/10.1016/j.jgg.2016.11.003
Jin TT, Liu P, Zhang ZX, Xu GD, Zhao LL (2009) Analysis of roots of soybean (Glycine max Merrill) treated with exogenous citric acid plus short-time aluminum stress by direct determination of FTIR spectrum. Guang Pu Xue Yu Guang Pu Fen Xi Guang Pu 29(2):367–371
Riaz M, Yan L, Wu X, Hussain S, Aziz O, Jiang C (2018) Mechanisms of organic acids and boron induced tolerance of aluminum toxicity: a review. Ecotoxicol Environ Saf 165:25–35. https://doi.org/10.1016/j.ecoenv.2018.08.087
Jiang Q, Luo Y, Chen L et al (2023) Identification of aluminum-resistant miRNAs and lncRNAs in Vitis quinquangularis and exploration of the related aluminum-tolerance mechanisms. Environ Exp Bot 206:105194. https://doi.org/10.1016/j.envexpbot.2022.105194
Zhang P, Zhou Y, Pan X et al (2023) Enhanced acidogenic fermentation from Al-rich waste activated sludge by combining lysozyme and sodium citrate pretreatment: perspectives of Al stabilization and enzyme activity. Sci Total Environ 864:161108. https://doi.org/10.1016/j.scitotenv.2022.161108
Mailloux RJ, Lemire J, Kalyuzhnyi S, Appanna V (2008) A novel metabolic network leads to enhanced citrate biogenesis in Pseudomonas fluorescens exposed to aluminum toxicity. Extremophiles 12(3):451–459. https://doi.org/10.1007/s00792-008-0150-1
Lemire J, Mailloux R, Auger C, Whalen D, Appanna VD (2010) Pseudomonas fluorescens orchestrates a fine metabolic-balancing act to counter aluminium toxicity. Environ Microbiol 12(6):1384–1390. https://doi.org/10.1111/j.1462-2920.2010.02200.x
Mossand G, Lelong E, Xing C et al (2023) Bis-catecholamide-based materials for uranium extraction. ChemPlusChem 88(5):e202300103. https://doi.org/10.1002/cplu.202300103
Lian J, Liu Y, Chen L, Li L, Ding D, Dai Z (2022) Facile synthesis of calcium peroxide modified mesoporous silica for enhanced uranium extraction from uranium tailings leachate. J Environ Chem Eng 10(6):108914. https://doi.org/10.1016/j.jece.2022.108914
Tykva R, Novák J, Podracká E, Popa K (2023) Bioaccumulation of uranium from waste water using different strains of Saccharomyces cerevisiae. Nukleonika. Published online 2009. Accessed 1 Dec 2023. https://www.semanticscholar.org/paper/Bioaccumulation-of-uranium-from-waste-water-using-Tykva-Nov%C3%A1k/64feafd139bfa89ef93bc84454d589c3ca1eef25
Tsuruta T (2006) Bioaccumulation of uranium and thorium from the solution containing both elements using various microorganisms. J Alloys Compd 408–412:1312–1315. https://doi.org/10.1016/j.jallcom.2005.04.131
Bontemps A, Conquet L, Elie C et al (2019) In vivo comparison of the phenotypic aspects and molecular mechanisms of two nephrotoxic agents, sodium fluoride and uranyl nitrate. Int J Environ Res Public Health 16(7):1136. https://doi.org/10.3390/ijerph16071136
Elmhiri G, Gloaguen C, Grison S et al (2018) DNA methylation and potential multigenerational epigenetic effects linked to uranium chronic low-dose exposure in gonads of males and females rats. Toxicol Lett 282:64–70. https://doi.org/10.1016/j.toxlet.2017.10.004
Chen J, Zhang M, Shu J et al (2023) Radiation-induced de novo defects in metal-organic frameworks boost CO2 sorption. J Am Chem Soc 145(43):23651–23658. https://doi.org/10.1021/jacs.3c07778
Zhou C, Wu Y, Sun Y, Wang Y, Wang X, Zhang X (2014) Impact of histology on efficacy of pemetrexed: pemetrexed in second-line setting and as maintenance therapy after first-line treatment in Chinese patients with advanced NSCLC. Zhonghua Zhong Liu Za Zhi 36(1):29–33
Wang W, Ma Y, He J, Qi H, Xiao F, He S (2019) Gene regulation for the extreme resistance to ionizing radiation of Deinococcus radiodurans. Gene 715:144008. https://doi.org/10.1016/j.gene.2019.144008
Anoop VM, Basu U, McCammon MT, McAlister-Henn L, Taylor GJ (2003) Modulation of citrate metabolism alters aluminum tolerance in yeast and transgenic canola overexpressing a mitochondrial citrate synthase. Plant Physiol 132(4):2205–2217. https://doi.org/10.1104/pp.103.023903
Jaiswal SK, Naamala J, Dakora FD (2018) Nature and mechanisms of aluminium toxicity, tolerance and amelioration in symbiotic legumes and rhizobia. Biol Fertil Soils 54(3):309–318. https://doi.org/10.1007/s00374-018-1262-0
Belimov AA, Shaposhnikov AI, Syrova DS et al (2020) The role of symbiotic microorganisms, nutrient uptake and rhizosphere bacterial community in response of pea (Pisum sativum L.) genotypes to elevated Al concentrations in soil. Plants 9(12):1801. https://doi.org/10.3390/plants9121801
Wang M, Qiu J, Tao X et al (2011) Effect of pH and ionic strength on U(IV) sorption to oxidized multiwalled carbon nanotubes. J Radioanal Nucl Chem 288(3):895–901. https://doi.org/10.1007/s10967-011-1018-x
Macaskie LE, Yong P, Doyle TC, Roig MG, Diaz M, Manzano T (1997) Bioremediation of uranium-bearing wastewater: Biochemical and chemical factors influencing bioprocess application. Biotechnol Bioeng 53(1):100–109. https://doi.org/10.1002/(SICI)1097-0290(19970105)53:1%3c100::AID-BIT13%3e3.0.CO;2-S
Im DK, Hong J, Gu B, Sung C, Oh MK (2020) 13C metabolic flux analysis of Escherichia coli engineered for gamma-aminobutyrate production. Biotechnol J 15(6):1900346. https://doi.org/10.1002/biot.201900346
Acknowledgements
This work was financially supported by the Independent Research Project of the China Academy of Radiation Protection (2022 Platform Open Fund Project of the National Key Laboratory of Radiation Environment and Health for Environmental Protection -4), the Environmental Protection Research Project of Hunan Province (HBKYXM-2023021), the National Natural Science Foundation of China (42377076), the Natural Science Foundation of Hunan Province (2023JJ50129), and the Basic Applied Research Special Project of Hengyang (202002042158).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jiang, F., Chen, L., Cheng, C. et al. Study on the prevention and control of uranium pollution by Deinococcus radiodurans overexpressing Cs gene. J Radioanal Nucl Chem 333, 927–950 (2024). https://doi.org/10.1007/s10967-023-09330-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-023-09330-4