Abstract
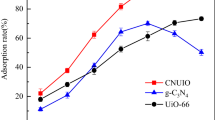
Based on the stable Zr6O4(OH)4 metal clusters, UiO-67 was first applied to the adsorption of U(VI), and its stability and adsorption performance for U(VI) were compared with UiO-66. As the organic ligand was lengthened, UiO-67 had a larger specific surface area and pore volume than UiO-66. The adsorption capacity of UiO-67 for uranium was 1.68 times that of UiO-66 under the same conditions. The chemical stability of UiO series decreased with the extension of organic ligands; for example, the crystalline structure of UiO-67 disintegrated in methanol, ethanol and water. Through the infrared spectra, adsorption isotherms and kinetics fitting, it is concluded that the carboxyl groups of UiO-67 participated in the chemisorption of U(VI), and uranyl ions adsorbed on UiO-67 were not desorbed with the collapse of UiO-67 crystal. This work represents the extension of organic chains reduces the chemical stability of MOFs but improves the adsorption performance of U(VI), which may be applied to predict the structural stability and adsorption capacity of other MOFs containing aromatic dicarboxylate organic linkers.











Similar content being viewed by others
Data availability
The authors declare that the data supporting the findings of this study are available within the paper. Should any raw data files be needed in another format they are available from the corresponding author upon reasonable request. Source data are provided with this paper.
References
Luo J, Chen J, Chen J, Ma J, Liu S, Tong X, Xiong J (2022) Aluminum vanadate microspheres is a simple but effective material for uranium extraction: performance and mechanism. J Solid State Chem 312:123237
Zhang Q, Zhang S, Zhao J, Wei P, Wang C, Liu P, Zhao X, Zeng K, Wu F, Liu Z (2021) Unexpected ultrafast and highly efficient removal of uranium from aqueous solutions by a phosphonic acid and amine functionalized polymer adsorbent. New J Chem 45:10777–10787
Jiang T, Zhang X, Xie X, Wu X, Luo C, Li M, Peng Y (2021) Effective capture of aqueous uranium using a novel magnetic goethite: properties and mechanism. J Solid State Chem 300:122236
Mei D, Liu L, Yan B (2023) Adsorption of uranium(VI) by metal-organic frameworks and covalent-organic frameworks from water. Coordin Chem Rev 475:214917
Tang N, Liang J, Niu C, Wang H, Luo Y, Xing W, Ye S, Liang C, Guo H, Guo J, Zhang Y, Zeng G (2020) Amidoxime-based materials for uranium recovery and removal. J Mater Chem A 8:7588–7625
Liu P, Ruan H, Li T, Wang H, Ma F (2023) Removal of U(VI) contamination from aqueous solution by ethylenediamine modified wet spinning polyacrylonitrile/amidoxime polyacrylonitrile composite fibers. J Radioanal Nucl Chem 332:3293–3303
Peng Y, Zhang X, Li M, Zhang Y, Wu X (2019) Application of metal-organic frameworks in adsorption and separation of uranium from water. Chem Ind Eng Prog 38:3227–3242
Cavka JH, Jakobsen S, Olsbye U, Olsbye U, Guillou N, Lamberti C, Bordiga S, Lillerud KP (2008) A new zirconium inorganic building brick forming metal organic frameworks with exceptional stability. J Am Chem Soc 130:13850–13851
Kandiah M, Nilsen MH, Usseglio S, Jakobsen S, Olsbye U, Tilset M, Larabi C, Quadrelli EA, Bonino F, Lillerud KP (2010) Synthesis and stability of tagged UiO-66 Zr-MOFs. Chem Mater 22:6632–6640
Ahmadijokani F, Mohammadkhani R, Ahmadipouya S, Shokrgozar A, Arjmand M (2020) Superior chemical stability of UiO-66 metal-organic frameworks (MOFs) for selective dye adsorption. Chem Eng J 399:125346
Dhaka S, Kumar R, Deep A, Kurade MB, Ji SW, Jeon BH (2019) Metal-organic frameworks (MOFs) for the removal of emerging contaminants from aquatic environments. Coordin Chem Rev 380:330–352
Wang X, Li L, Wang Y, Li J, Li J (2017) Exploiting the pore size and functionalization effect in UiO topology structures used for the separation of light hydrocarbons. CrystEngComm 19:1729–1737
Sini K, Bourgeois D, Idouhar M, Carboni M, Meyer D (2019) Metal-organic frameworks cavity size effect on the extraction of organic pollutants. Mater Lett 250:92–95
Carboni M, Abney CW, Liu S, Lin W (2013) Highly porous and stable metal-organic frameworks for uranium extraction. Chem Sci 4:2396–2402
Luo B, Yuan L, Chai Z, Shi W, Tang Q (2016) U(VI) capture from aqueous solution by highly porous and stable MOFs: UiO-66 and its amine derivative. J Radioanal Nucl Chem 307:269–276
Chen L, Bai Z, Zhu L, Zhang L, Cai Y, Li Y, Liu W, Wang Y, Chen L, Diwu J, Wang J, Chai Z, Wang S (2017) Ultrafast and efficient extraction of uranium from seawater using an amidoxime appended metal-organic framework. ACS Appl Mater Interfaces 9(32446):32451
Ma L, Gao J, Huang C, Xu X, Xu L, Ding R, Bao H, Wang Z, Xu G, Li Q, Deng P, Ma H (2021) UiO-66-NH-(AO) MOFs with a new ligand BDC-NH-(CN) for efficient extraction of uranium from seawater. ACS Appl Mater interfaces 13:57831–57840
Zhao B, Yuan L, Wang Y, Duan T, Shi W (2021) Carboxylated UiO-66 tailored for U(VI) and Eu(III) trapping: from batch adsorption to dynamic column separation. ACS Appl Mater Interfaces 13:16300–16308
Wang X, Chen L, Bai Z, Zhang D, Guan J, Zhang Y, Shi C, Diwu J (2020) In vivo uranium sequestration using a nanoscale metal-organic framework. Angew Chem Int Ed 60:1646–1650
Yuan Y, Feng S, Feng L, Yu Q, Liu T, Wang N (2020) A Bio-inspired nano-pocket spatial structure for targeting uranyl capture. Angew Chem Int Ed 59:4262–4268
Yu Q, Yuan Y, Wen J, Zhao X, Zhao S, Wang D, Li C, Wang X, Wang N (2019) A universally applicable strategy for construction of anti-biofouling adsorbents for enhanced uranium recovery from seawater. Adv Sci 6:1900002
Yang P, Liu Q, Liu J, Zhang H, Li Z, Li R, Liu L, Wang J (2017) Interfacial growth of a metal-organic framework (UiO-66) on functionalized graphene oxide (GO) as a suitable seawater adsorbent for extraction of uranium(VI). J Mater Chem A 5:17933–21794
Peng Y, Zhang X, Wu X, Li M, Zhang Y, Zhou C, Hua Y (2022) Synthesis of core-shell magnetic metal organic frameworks composite for efficient uranium(VI) removal. New J Chem 46:503–7511
Fotovat H, Khajeh M, Oveisi A, Ghaffari-Moghaddam M, Daliran S (2018) A hybrid material composed of an amino-functionalized zirconium-based metal-organic framework and a urea based porous organic polymer as an efficient sorbent for extraction of uranium(VI). Microchim Acta 185:469–476
Liu T, Zhang X, Wang H, Chen M, Yuan Y, Zhang R, Xie Z, Liu Y, Zhang H, Wang N (2021) Photothermal enhancement of uranium capture from seawater by monolithic MOF-bonded carbon sponge. Chem Eng J 412:128700
Yuan L, Tian M, Lan CX, Wang CZ, Gibson J, Shi W (2018) Defect engineering in metal-organic frameworks: a new strategy to develop applicable actinide sorbents. Chem Commun 54:370–373
Zhang Z, Zhao Y, Zhang B, Shen W (1984) Uranium geochemistry. Atomic Energy Press, Beijing
Guo Y, Jia Z, Shi Q, Liu Z, Wang X, Li L (2019) Zr (IV)-based coordination porous materials for adsorption of Copper(II) from water. Micropor Mesopor Mat 285:215–222
Al-Jadir TM, Siperstein FR (2018) The influence of the pore size in Metal−Organic Frameworks in adsorption and separation of hydrogen sulphide: a molecular simulation study. Micropor Mesopor Mat 271:160–168
Zhong G, Liu D, Zhang J (2018) Incorporation of functional groups expands the applications of UiO-67 for adsorption, catalysis and thiols detection. ChemistrySelect 3:7066–7080
DeCoste JB, Peterson GW, Jasuja H, Glover G, Huang Y, Walton K (2013) Stability and degradation mechanisms of metal-organic frameworks containing the Zr6O4(OH)4 secondary building unit. J Mater Chem A 1:5642–5650
Øien-Ødegaard S, Bouchevreau B, Hylland K, Wu L, Blom R, Grande C, Olsbye U, Tilset M, Lillerud K (2016) UiO-67-type metal-organic frameworks with enhanced water stability and methane adsorption capacity. Inorg Chem 55:1986–1991
Ebrahim AM, Levasseur B, Bandosz TJ (2013) Interactions of NO2 with Zr-based MOF: effects of the size of organic linkers on NO2 adsorption at ambient conditions. Langmuir 29:168–174
Schaate A, Roy P, Godt A, Lippke J, Waltz F, Wiebcke M, Behrens P (2011) Modulated synthesis of Zr-Based metal-organic frameworks: from nano to single crystals. Chem Eur J 17:6643–6665
Wabaidur SM, Khan MA, Siddiqui R, Otero M, Jeon BH, Alothman ZA, Hakami AAH (2020) Oxygenated functionalities enriched MWCNTs decorated with silica coated spinel ferrite-A nanocomposite for potentially rapid and efficient de-colorization of aquatic environment. J Mol Liq 317:113916
Katz MJ, Brown ZJ, Colón YJ, Siu PW, Scheidt KA, Snurr RQ, Hupp JT, Farha OK (2013) A facile synthesis of UiO-66, UiO-67 and their derivatives. Chem Commun 49:9449–9451
Howarth AJ, Liu YY, Li P, Li Z, Wang T, Hupp JT, Farha OK (2016) Chemical, thermal and mechanical stabilities of metal-organic frameworks. Nat Rev Mater 1:1–15
Feng M, Zhang P, Zhou H, Sharma V (2018) Water-stable metal-organic frameworks for aqueous removal of heavy metals and radionuclides: a review. Chemosphere 209:783–800
Zhang X, Wang J, Li R, Dai Q, Liu L (2013) Removal of uranium(VI) from aqueous solutions by surface modified magnetic Fe3O4 particles. New J Chem 37:3914–4391
Yang Q, Wang Y, Wang J, Liu F, Hu N, Pei H, Yang W, Li Z, Suo Y, Wang J (2018) High effective adsorption/removal of illegal food dyes from contaminated aqueous solution by Zr-MOFs (UiO-67). Food Chem 254:241–248
Deshmukh P, Sar SK, Jindal MK (2023) Plant mediated magnetic nano composite as promising scavenger’s radionuclides for the efficient remediation in aqueous medium. Chemosphere 312:137246
Deshmukh P, Sar SK, Jindal MK, Ray T (2023) Magnetite based green bio composite for uranium exclusion from aqueous solution. J Radioanal Nucl Chem 332:297–310
Kamari A, Ngah WSW, Chong MY, Cheah M (2009) Sorption of acid dyes onto GLA and H2SO4 cross-linked chitosan beads. Desalination 249:1180–1189
Tan L, Wang J, Liu Q, Sun Y, Jing X, Liu L, Liu J, Song D (2015) The synthesis of a manganese dioxide-iron oxide-graphene magnetic nanocomposite for enhanced uranium(VI) removal. New J Chem 39:868–876
Acknowledgements
This work was supported by the Science and Technology Innovation Platform Plan of Hengyang (Grant No. 202150083887) and the Hunan Students' Innovation and Entrepreneurship Training Program (Grant No. S201910555107).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Peng, Y., Zhang, X., Zhang, Y. et al. Stability and adsorption performance of UiO-67 for uranium(VI) in solution. J Radioanal Nucl Chem 333, 305–315 (2024). https://doi.org/10.1007/s10967-023-09279-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-023-09279-4