Abstract
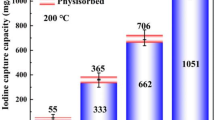
The Cu2O/Cu modified shungite (ShC) absorbent (Cu2O/Cu-ShC) was synthesized by hydrothermal method, benefiting from the mineral backbone and Cu2O/Cu particles, Cu2O/Cu-ShC can remove ~ 96% of I− within 60 min, the batch experiments showed that the I− adsorption process follows pseudo-second-order model and Langmuir model with maximum adsorption capacity of 10.99 mg/g. The high binding affinity between Cu+ and I− contributes effective removal of I− and excellent selectivity at high concentrations of competing ions. This work highlights the feasibility of Cu2O/Cu-ShC for the radioactive iodine separation, and provides a promising sorbent material for large-scale purification of radioactive iodine anions in wastewater.









Similar content being viewed by others
References
Chen YY et al (2018) One-step synthesis of Ag2O@Mg(OH)2 nanocomposite as an efficient scavenger for iodine and uranium. Colloid Interface Sci 510:280–291
Sag AA, Kazaure HS, Kelley CE (2022) Role of thyroid RFA in the treatment of autonomously functioning thyroid nodules. Tech Vasc Interv Radiol 25(2):100823
Sellem A et al (2020) Role and effectiveness of radioactive-iodine therapy for the treatment of Grave’s disease. Pan Afr Med J 36:341
Boucher A et al (2021) Canadian consensus statement on the management of radioactive iodine-resistant differentiated thyroid cancer. Oral Oncol 121:9
Tulchinsky M (2019) Selective history of radioactive iodine in medicine: inexactitudes no longer. Ejso 45(4):711–712
Truesdale VW, Nausch G, Baker A (2001) The distribution of iodine in the Baltic Sea during summer. Mar Chem 74(2):87–98
Kulyukhin SA et al (2019) Combined filter for purifying a radioactive steam-gas mixture. At Energ 125(4):262–264
Guo Z, Chu TW (2022) Selective separation of uranium, zirconium and iodine from various fission products by 1-butyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imide and phosphoramide-functionalized ionic liquid. J Radioanal Nucl Chem 331(9):3905–3913
Li C et al (2018) Efficient and rapid adsorption of iodide ion from aqueous solution by porous silica spheres loaded with calcined Mg–Al layered double hydroxide. J Taiwan Inst Chem Eng 85:193–200
Park JE et al (2020) A functionalized nanocomposite adsorbent for the sequential removal of radioactive iodine and cobalt ions in aqueous media. Korean J Chem Eng 37(12):2209–2215
Zhang W et al (2019) Cross-linked chitosan microspheres: an efficient and eco-friendly adsorbent for iodide removal from waste water. Carbohydr Polym 209:215–222
Wang Y et al (2023) Influence of organic dispersant on nuclear grade anion exchange resin and their interaction behavior. J Nucl Sci Technol 60(2):140–152
Theiss FL et al (2014) A review of the removal of anions and oxyanions of the halogen elements from aqueous solution by layered double hydroxides. J Colloid Interface Sci 417:356–368
Rizescu C et al (2021) Hydrodeoxygenation of benzyl alcohol on transition-metal-containing mixed oxides catalysts derived from layered double hydroxide precursors. Catal Today 366:235–244
Mondal R et al (2020) Efficient silver release from ion exchange silver dressings in biologically relevant media. Wounds-A Compend Clin Res Pract 32(1):22–29
Kim T et al (2020) Comment on “Visible-light-driven, hierarchically heterostructured, and flexible silver/bismuth oxyiodideititania nanofibrous membranes for highly efficient water disinfection” by Song et al. J Colloid Interface Sci 566:513–514
Mailen JC, Horner DE (2017) Removal of iodine from reactor fuel solutions as insoluble PdI2. Nucl Technol 33(3):260–263
Azat S et al (2020) Synthesis of biosourced silica-Ag nanocomposites and amalgamation reaction with mercury in aqueous solutions. C R Chim 23(1):77–92
Jiang J et al (2021) Efficient adsorption of radioactive iodide by copper/palygorskite composite. J Inorg Mater 36(8):856–864
Ni GX et al (2020) Nanoscale infrared spectroscopy and imaging of catalytic reactions in Cu2O Crystals. ACS Photon 7(3):576–580
Rodriguez-Martinez Y et al (2022) One-step formation of plasmonic Cu nanodomains in p-type Cu2O matrix films for enhanced photoconversion of n-ZnO/p-Cu2O heterojunctions. ACS Appl Electron Mater 4(11):5527–5537
Zheng JH, Liu XY, Zhang L (2020) Design of porous double-shell Cu2O@CuCo2O4 Z-scheme hollow microspheres with superior redox property for synergistic photocatalytic degradation of multi-pollutants. Chem Eng J 389:10
Zhang X et al (2017) Efficient adsorption of radioactive iodide ion from simulated wastewater by nano Cu2O/Cu modified activated carbon. Chem Eng J 322:129–139
Lefèvre G et al (2000) Sorption of iodide on cuprite (Cu2O). Langmuir 16(10):4519–4527
Skrypnik L et al (2021) A study of the antioxidant, cytotoxic activity and adsorption properties of karelian shungite by physicochemical methods. Antioxidants 10(7):10
Goryunov AS et al (2023) Structural and colloid effects of interaction between shungite carbon nanoparticles and linoleic fatty acid. Curr Nanosci 19(1):68–75
Sukhinina NS et al (2022) Structural features and sorption properties of mesoporous carbon material prepared from natural shungite. Inorg Mater 58(10):1114–1121
Skorobogatov GA, Ashmarova YA, Rebrova AG (2017) Transformations of shungite in aqueous media (pH from 1 to 12). Russ J Appl Chem 90(1):113–119
Ermagambet BT et al (2017) Fischer-Tropsch synthesis using cobalt catalyst containing modified shungite. Solid Fuel Chem 51(2):101–106
Chou NH et al (2018) Carbon-rich shungite as a natural resource for efficient Li-ion battery electrodes. Carbon 130:105–111
Zhang D et al (2022) Cu/CuOx@C composite as a high-efficiency electrocatalyst for oxygen reduction reactions. Catalysts 12(12):12
Takeuchi Y, Ohkubo T (2023) Polymer template synthesis of CuOx/clay nanocomposites with controllable CuOx formation. ChemistrySelect 8(31):9
Wang HJ et al (2023) An electrochemiluminescence immunosensor based on multipath signal catalytic amplification integrated in a Cu2+-PEI-Pt/AuNC nanocomposite. Analyst 148(14):3354–3358
Li YT et al (2020) Adsorption behaviors and mechanism of graphene oxide for silver complex anion removal. Appl Surf Sci 529:9
Zhang L, Jaroniec M (2017) SBA-15 templating synthesis of mesoporous bismuth oxide for selective removal of iodide. J Colloid Interface Sci 501:248
Sha X et al (2020) Mussel-inspired preparation of MXene-PDA-Bi6O7 composites for efficient adsorptive removal of iodide ions. J Environ Chem Eng 8(5):104261
Li BS et al (2023) A chitosan film implanted with g-C3N4@Ag nanosheets and in-suit formed AgCl nanoparticles for efficient iodide removal. Chem Eng J 470:11
Benkhaled BT et al (2023) Novel biocompatible trianglamine networks for efficient iodine capture. ACS Appl Mater Interfaces 15(36):42942–42953
Yu WL et al (2022) Preparation of halloysite/Ag2O nanomaterials and their performance for iodide adsorption. Minerals 12(3):11
Babujohn NA, Eluri A (2023) Viologen-functionalized magnetic material for the removal of iodine and benzanthracene in an aqueous solution. Environ Sci Pollut Res 30(27):69991–70010
Zhu H et al (2023) In-site interface growth of bismuth-based hydrothermal carbon using collagen fiber for selective removal of iodide ion from wastewater. Colloids Surf A Physicochem Eng Aspects 664:8
Du WJ et al (2020) Efficient capture of volatile iodine by thiophene-containing porous organic polymers. ACS Appl Polym Mater 2(11):5121–5128
Haq Z et al (1980) Sorption of iodide on copper. Environ Sci Technol 14(9):1106–1110
Yan CH et al (2023) Cuprous oxide-based cationic hydrogel by the integration of enrichment and immobilization of radioiodine (I−, IO3−) in Aqueous Solution. ACS Appl Mater Interfaces 15(23):28135–28148
Liu Y et al (2016) An investigation into the use of cuprous chloride for the removal of radioactive iodide from aqueous solutions. J Hazard Mater 302:82–89
Pedersen DB, Wang S, Liang SH (2008) Charge-transfer-driven Diffusion processes in Cu@Cu-oxide core–shell nanoparticles. J Phys Chem C 112(24):8819–8826
Wu CK et al (2006) Quantitative analysis of copper oxide nanoparticle composition and structure by x-ray photoelectron spectroscopy. Chem Mater 18(25):6054–6058
Ai ZH et al (2009) Interfacial hydrothermal synthesis of Cu@Cu2O core–shell microspheres with enhanced visible-light-driven photocatalytic activity. J Phys Chem C 113(49):20896–20902
Acknowledgements
This work was supported by the National Natural Science Foundation of China (NSFC, Grant Number 42061134018) and the Russian Science Foundation (RSF, Grant Number 21-47-00019), the Project of Spent Fuel Reprocessing (KY20007), the Project of Science and Technology Department of Sichuan Province (No. 2021JDTD0019) and the Doctoral Research Foundation Project of Southwest University of Science and Technology (No. 22zx7173).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tang, H., Zhang, M., Ran, F. et al. Efficient immobilization of iodide from aqueous solution by Cu2O/Cu modified shungite. J Radioanal Nucl Chem 333, 545–556 (2024). https://doi.org/10.1007/s10967-023-09267-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-023-09267-8