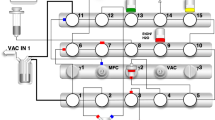
Abstract
In this study, the optimum conditions for the synthesis of [68Ga]Ga-PSMA-11 were determined for the first time using the Quality by Design (QbD). The response surface methodology was used to estimate the effect of input variables (PSMA-11 concentration, time, reaction temperature, and pH) on the responses calculated as the percentage of the radiochemical purity and the RR configuration. Since using QbD principles in the production of radiopharmaceuticals is not common, this study will help increase the number of applications in this field and ensure quality in radiopharmaceutical manufacturing, allowing decreased cost and the number of experiments.
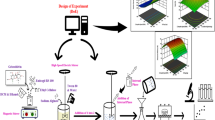
Graphical abstract




Similar content being viewed by others
References
Evangelista L, Maurer T, van der Poel H et al (2022) [68Ga]Ga-PSMA versus [18F] PSMA positron emission tomography/computed tomography in the staging of primary and recurrent prostate cancer. A systematic review of the literature. Eur Urol Oncol 5(3):273–282
Rasul S, Haug AR (2022) Clinical applications of PSMA PET examination in patients with prostate cancer. Cancers (Basel) 14:3768. https://doi.org/10.3390/cancers14153768
Lenzo NP, Meyrick D, Turner JH (2018) Review of gallium-68 PSMA PET/CT imaging in the management of prostate cancer. Diagnostics 8:16
Eiber M, Weirich G, Holzapfel K et al (2016) Simultaneous (68)Ga-PSMA HBED-CC PET/MRI improves the localization of primary prostate cancer. Eur Urol 70:829–836. https://doi.org/10.1016/j.eururo.2015.12.053
Kesch C, Kratochwil C, Mier W et al (2017) 68Ga or 18F for prostate cancer imaging? J Nucl Med 58:687–688
Lindenberg L, Choyke P, Dahut W (2016) Prostate cancer imaging with novel PET tracers. Curr Urol Rep 17:1–8
Schwarzenboeck SM, Rauscher I, Bluemel C et al (2017) PSMA ligands for PET imaging of prostate cancer. J Nucl Med 58:1545–1552
Eder M, Neels O, Müller M et al (2014) Novel preclinical and radiopharmaceutical aspects of [68Ga]Ga-PSMA-HBED-CC: a new PET tracer for imaging of prostate cancer. Pharmaceuticals 7:779–796
Nanabala R, Anees MK, Sasikumar A et al (2016) Preparation of [[68Ga]PSMA-11 for PET–CT imaging using a manual synthesis module and organic matrix based 68Ge/68Ga generator. Nucl Med Biol 43:463–469
Calderoni L, Farolfi A, Pianori D et al (2020) Evaluation of an automated module synthesis and a sterile cold kit–based preparation of 68Ga-PSMA-11 in patients with prostate cancer. J Nucl Med 61:716–722
Beheshti M, Paymani Z, Brilhante J et al (2018) Optimal time-point for 68Ga-PSMA-11 PET/CT imaging in assessment of prostate cancer: feasibility of sterile cold-kit tracer preparation? Eur J Nucl Med Mol Imaging 45:1188–1196
Yu LX (2008) Pharmaceutical quality by design: product and process development, understanding, and control. Pharm Res 25:781–791. https://doi.org/10.1007/s11095-007-9511-1
Guideline ICHHT (2009) Pharmaceutical development. Q8 (2R) as revised august
International Council for Harmonisation (2015) ICH guideline Q10 on pharmaceutical quality system. Eur Med Agency 44:1–20
ICH (2005) ICH guideline Q9 on quality risk management. Regul ICH 44:1–20
Gundogdu E, Demir ES, Özgenç E et al (2020) Applying quality by design principles in the development and preparation of a new radiopharmaceutical: technetium-99m-imatinib mesylate. ACS Omega 5:5297–5305
Lange R, Ter Heine R, Van Der Gronde T et al (2016) Applying quality by design principles to the small-scale preparation of the bone-targeting therapeutic radiopharmaceutical rhenium-188-HEDP. Eur J Pharm Sci 90:96–101
Beg S, Chaudhary V, Sharma G et al (2016) QbD-oriented development and validation of a bioanalytical method for nevirapine with enhanced liquid–liquid extraction and chromatographic separation. Biomed Chromatogr 30:818–828
Bezerra MA, Santelli RE, Oliveira EP et al (2008) Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 76(5):965–977
Mourabet M, El Rhilassi A, El Boujaady H et al (2017) Use of response surface methodology for optimization of fluoride adsorption in an aqueous solution by Brushite. Arab J Chem 10:S3292–S3302
Politis SN, Colombo P, Colombo G, Rekkas DM (2017) Design of experiments (DoE) in pharmaceutical development. Drug Dev Ind Pharm 43:889–901
Yousry C, Farrag NS, Amin AM (2023) Radiolabeling of statistically optimized nanosized atorvastatin suspension for liver targeting and extensive imaging of hepatocellular carcinoma. J Drug Deliv Sci Technol 80:104171
Pehlivanoglu H, Ocak M, Caglar-Andac S (2022) Development and validation of a UV-Radio-HPLC method to assess chemical and radiochemical purity of [[68Ga]Ga-PSMA-11. Appl Radiat Isot 190:110487. https://doi.org/10.1016/j.apradiso.2022.110487
Schultz MK, Mueller D, Baum RP et al (2013) A new automated NaCl based robust method for routine production of gallium-68 labeled peptides. Appl Radiat Isot 76:46–54
Mueller D, Breeman WAP, Klette I et al (2016) Radiolabeling of DOTA-like conjugated peptides with generator-produced 68Ga and using NaCl-based cationic elution method. Nat Protoc 11:1057–1066
Velikyan I (2015) 68Ga-based radiopharmaceuticals: production and application relationship. Molecules 20:12913–12943
Amor-Coarasa A, Schoendorf M, Meckel M et al (2016) Comprehensive quality control of the ITG 68Ge/68Ga generator and synthesis of 68Ga-DOTATOC and 68Ga-PSMA-HBED-CC for clinical imaging. J Nucl Med 57:1402–1405
Maruk AY, Larenkov AA (2020) Determination of ionic 68Ga impurity in radiopharmaceuticals: major revision of radio-HPLC methods. J Radioanal Nucl Chem 323:189–195
Rathore AS, Winkle H (2009) Quality by design for biopharmaceuticals. Nat Biotechnol 27:26–34
Kaur P, Garg T, Rath G et al (2016) Development, optimization and evaluation of surfactant-based pulmonary nanolipid carrier system of paclitaxel for the management of drug resistance lung cancer using box-behnken design. Drug Deliv 23:1912–1925
Sharma D, Maheshwari D, Philip G et al (2014) Formulation and optimization of polymeric nanoparticles for intranasal delivery of lorazepam using box-behnken design: in vitro and in vivo evaluation. Biomed Res Int 2014:156010
Luurtsema G, Pichler V, Bongarzone S et al (2021) EANM guideline for harmonisation on molar activity or specific activity of radiopharmaceuticals: impact on safety and imaging quality. EJNMMI Radiopharm Chem 6:34. https://doi.org/10.1186/s41181-021-00149-6
Eppard E, Homann T, de la Fuente A et al (2017) Optimization of labeling PSMAHBED with ethanol-postprocessed 68Ga and its quality control systems. J Nucl Med 58:432–437
Acknowledgements
We would like to thank Istanbul University-Cerrahpasa, Nuclear Medicine Department for the support in obtaining the 68Ga radionuclide and Modular-Lab Standard module (Eckert & Ziegler).
Funding
This study was supported by the Research Fund of Istanbul University with Project No: TYL-2019–33814.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pehlivanoglu, H., Ocak, M. & Caglar-Andac, S. Application of response surface methodology and quality by design to [68Ga]Ga-PSMA-11 preparation. J Radioanal Nucl Chem 333, 43–51 (2024). https://doi.org/10.1007/s10967-023-09246-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-023-09246-z