Abstract
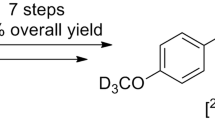
Celecoxib are COX2 inhibitors approved to reduce the COX2 level. Celecoxib and its major metabolites labeled with deuteriums are applied for drug metabolism studies. [2H4] celecoxib was prepared from [2H4] 4-acetamidobenzenesolfonyl through amination, hydrosis, diazotization, reduction and cyclization. After bromination and hydrolysis reaction, [2H4] hydroxy celecoxib was obtained and further oxidized by tetrabutylammonium permanganate to afford [2H4] celecoxib carboxylic acid. The title compounds showed remarkable chemical purity and isotope abundance which can be utilized in pharmacokinetic research.





Similar content being viewed by others
References
Simmons DL, Botting RM, Hla T (2004) Cyclooxygenase isozymes: the biology of prostaglandin synthesis and inhibition. Pharmacol Rev 56:387–437
O’Banion MK, Sadowski HB, Winn V, Young DA (1991) A serum- and glucocorticoid-regulated 4-kilobase mRNA encodes a cyclooxygenase-related protein. J Biol Chem 266(34):23261–23267
Kujubu DA, Fletcher BS, Varnum BC, Lim RW, Herschman HR (1991) TIS10, a phorbol ester tumor promoter-inducible mRNA from Swiss 3T3 cells, encodes a novel prostaglandin synthase/cyclooxygenase homologue. J Biol Chem 266(20):12866–12872
Goldstien JL, Silverstein FE, Agrawal NM et al (2000) Reduced risk of upper gastrointestinal ulcer complications with celecoxib, a novel COX-2 inhibitor. Am J Gastroenterol 95(6):1681–1690
Vane JR, Bakhe YS, Botting RM (1998) CYCLOOXYGENASES 1 and 2. Ann Rev Pharmacol Toxicol 38:97–120
Chandrasekharan NV, Dai H, Roos KLT et al (2002) COX-3, a cyclooxygenase-1 variant inhibited by acetaminophen and other analgesic/antipyretic drug: cloning, structure, and expression. Proc Natl Acad Sci USA 99(21):13926–13931
Graul A, Martel AM, Castañer J (1997) Celecoxib. Drugs Fut 22:711–714
Davies NM, McLachlan AJ, Day RO, Williams KM (2000) Clinical pharmacokinetics and pharmacodynamics of celecoxib: a selective cyclo-oxygenase-2 inhibitor. Clin Pharmacokinet 38:225–242
Paulson SK, Hribar JD, Liu NW et al (2000) Metabolism and excretion of [14C] celecoxib in healthy male volunteers. Drug Metab Dispos 28:308–314
Tang C, Shou M, Mei Q, Rushmore TH, Rodrigues AD (2000) Major role of human liver microsomal cytochrome P450 2C9 (CYP2C9) in the oxidative metabolism of celecoxib, a novel cyclooxygenase-II inhibitor. J Pharmacol Exp Ther 293:453–459
Sandberg M, Yasar U, Strömberg P, Höög JO, Eliasson E (2002) Oxidation of celecoxib by polymorphic cytochrome P450 2C9 and alcohol dehydrogenase. Br J Clin Pharmacol 54:423–429
Talley JJ, Penning TD, Collins PW, et al (1995) Substituted pyrazolyl benzenesulfonamides. US5466823
O′ Shea P, Tillyer RD, Wang X, et al (2000) Synthesis of 4-[5-substituted or unsubstituted phenyl)-3-substituted-1H-pyrazol-1-yl]benzenesulfonamides. US6150534
Penning TD, Talley JJ, Bertenshaw SR et al (1997) Synthesis andbiological evaluation of the 1,5-diarylpyrazole class of cy-clooxygenase-2 inhibitors: identification of 4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl] benzene-sulfonamide. J Med Chem 40(9):1347–1365
Reddy M, Ramana V, Bell SC (2003) Process for the preparation of 1, 5-diarylpyrazoles. WO03024400A2
Reddy AR, Sampath A, Goverdhan G et al (2009) A improved and scalable process for celecoxib: a selective cyclooxyagenase-2 inhibitor. Org Process Res 12(13):98–101
Lynette MOH (2006) Synthesis of celecoxib via 1, 3-dipolar cycloaddition. Tetrahedron Lett 47:7943–7946
Steven MG, Reuben M, Nigel AS (2011) A novel three-step synthesis of celecoxib via palladium-catalyzed direct arylation. Tetrahedron Lett 52:6000–6002
Abdellatif KRA, Chowdhury MA, Dong Y et al (2008) Diazen-1-ium-1,2-diolated nitric oxide donor ester prodrugs of 5-(4-hydroxymethylphenyl)-1-(4-aminosulfonylphenyl)-3-trifluoromethyl-1H-pyrazole and its methanesulfonyl analog: synthesis, biological evaluation and nitric oxide release studies. Bioorg Med Chem 16(14):9694–9698
Takashima-Hirano M, Takashima T, Katayama Y et al (2011) Efficient sequential synthesis of PET Probes of the COX-2 inhibitor [11C]celecoxib and its major metabolite [11C]SC-62807 and in vivo PET evaluation. Bioorg Med Chem 19(9):2997–3004
Shi L, Li C, Chen R et al (2010) Synthesis of deuterium-labelled fosamprenavir calcium. J Labelled Com Radiopharm 53(3):147–151
Chen LQ, Li J, Shen YZ (2009) Preparation of losartan 5-carboxylic acid and use thereof, US20090885A1
He M, Li J, Tian L (2019) Synthesis of deuterium-labeled (3R, 5S)-fluvastatin and (3S, 5R)-fluvastatin. J Radioanal Nucl Chem 319:263–269
Prabhakaran J, Majo JV, Simpson RN et al (2005) Synthesis of [11C] celecoxib: a potential PET probe for imaging COX-2 expression. J Label Compd Radiopharm 48:887–895
Acknowledgements
This work was supported by the CNPC Research Institutes Safety & Environmental Technology Programme (Grant No. 2021DJ6605), National Natural Science Foundation of China (Grant No. 41472124), PetroChina Innovation Foundation (Grant Nos. 2015D-5006-0210 and 2016D-5007-0702) and Nature Science Foundation of Hubei Province (Grant No. 2016CFB178).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest in this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
He, M., Xiang, G., Duan, L. et al. Synthesis of deuterium-labeled celecoxib and its metabolites. J Radioanal Nucl Chem 332, 5045–5050 (2023). https://doi.org/10.1007/s10967-023-09242-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-023-09242-3