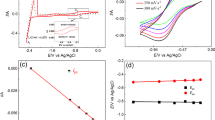
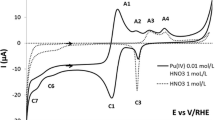
Abstract
To enhance the extraction of remaining fissile nuclides from spent nuclear fuel through pyrochemical reprocessing, the operational lifetime of such fuels can be extended, ultimately leading to increased cost efficiency and a reduction in the amount of radioactively contaminated waste generated by fast-neutron reactors. Understanding the electrochemical behavior of fissile nuclides in LiCl–KCl is pivotal to the success of molten salt electrorefining pyrochemical reprocessing. In this pursuit, a comprehensive study of Pu(III) salt was conducted to comprehend its electrochemical characteristics within molten chloride salt mixtures. To achieve this, PuCl3 was meticulously prepared by reacting PuO2 with HCl in a LiCl–KCl mixture. Subsequently, we investigated the reduction mechanism, the diffusion coefficient of Pu(III) (DPu(III)), and the apparent standard reduction potential of Pu(III)/Pu(0) (E0*Pu(III)/Pu(0)) in situ, using a Mo working cathode. Our findings revealed that Pu(III) undergoes a single-step reduction to Pu(0), involving the exchange of three electrons. Furthermore, the rate of diffusion governs the reduction of Pu(III) at the Mo cathode. The relationship between the diffusion coefficient and temperature was described by lnD = − 5.51 to 4244.2/T, with an activation energy of 35.28 kJ/mol. Additionally, we examined the temperature-dependent variations of E0*Pu(III)/Pu(0) and the Gibbs free energy of formation for PuCl3 (ΔGPuCl3). These dependencies were found to be E0*Pu(III)/Pu(0) = − 3.194 + 6.4 × 10−4 T and ΔGPuCl3 = − 924.5 + 0.185 T, respectively.




Similar content being viewed by others
References
Quan M, Liu Q, Liu Y, Zhang Z, Dai Y, Wang Y, Cao X, Cheng Z-p, Wang Y, Liu Y (2022) Electroextraction and thermochemistry of fission element gadolinium on plumbum electrode in molten salt. Sep Purif Technol 284:119413. https://doi.org/10.1016/j.seppur.2021.119413
Nawada HP, Fukuda K (2004) Role of pyro-chemical processes in advanced fuel cycles. J Phys Chem Solids 66:647–651. https://doi.org/10.1016/j.jpcs.2004.07.022
Masset P, Konings RJ, Malmbeck R, Serp J, Glatz J-P (2005) Thermodynamic properties of ternary Me-Ga-In (Me= La, U) alloys in a fused Ga-In/LiCl-KCl system. J Chem Thermodyn 344(1–3):173–179. https://doi.org/10.1016/j.jct.2018.10.014
Im HJ, Yeon JW (2018) Comparison of the spectroscopic characteristics of uranium status when U (III) in LiCl-KCl eutectic melt is leached out with water and ionic liquid. J Lumin 196:302–305. https://doi.org/10.1016/j.jlumin.2017.12.037
Zhang J (2014) Electrochemistry of actinides and fission products in molten salts—data review. J Nucl Mater 447:271–284. https://doi.org/10.1016/j.jnucmat.2013.12.017
Sakamura Y, Shirai O, Iwai T, Suzuki Y (2001) Distribution behavior of Pu and americium in LiCl–KCl eutectic/liquid cadmium systems. J Alloys Compd 321(1):76–83. https://doi.org/10.1016/S0925-8388(01)00973-2
Can Y, Acar BB (2022) Nuclear proliferation resistance assessment of fuel cycles closed with complete co-processing of spent fuel. Prog Nucl Energ 150:104297. https://doi.org/10.1016/j.pnucene.2022.104297
Adamov E, Mochalov YS, Rachkov V, Khomyakov YS, Shadrin AY, Kascheev V, Khaperskaya A (2021) Spent nuclear fuel reprocessing and nuclear materials recycling in two-component nuclear energy. At Energy 130(1):29–35. https://doi.org/10.1007/s10512-021-00769-w
Caravaca C, Laplace A, Vermeulen J, Lacquement J (2008) Determination of the E-pO2− stability diagram of Pu in the molten LiCl–KCl eutectic at 450°C. J Nucl Mater 377(2):340–347. https://doi.org/10.1016/j.jnucmat.2008.01.031
Castrillejo Y, Bermejo M, Pardo R, Martınez A (2002) Use of electrochemical techniques for the study of solubilization processes of cerium–oxide compounds and recovery of the metal from molten chlorides. J Electrochem Soc 522:124–140. https://doi.org/10.1016/S0022-0728(02)00717-9
Cordoba G, Caravaca C (2004) An electrochemical study of samarium ions in the molten eutectic LiCl + KCl. J Electrochem Soc 572:145–151. https://doi.org/10.1016/j.jelechem.2004.05.029
Castrillejo Y, Bermejo M, Barrado E, Martínez A (2006) Electrochemical behaviour of erbium in the eutectic LiCl–KCl at W and Al electrodes. Electrochim Acta 51(10):1941–1951. https://doi.org/10.1016/j.electacta.2005.07.004
Wu X, Wang Y, Hua Z, Weng H, Liu H, Zhao Z (2022) Electrochemical behavior and separation of dysprosium from molten NaCl-KCl eutectic by co-reduction with aluminum. Sep Purif Technol 301:122068. https://doi.org/10.1016/j.seppur.2022.122068
Jiao S, Zhu H (2011) An investigation into the electrochemical recovery of rare earth ions in a CsCl-based molten salt. J Hazard Mater 189(3):821–826. https://doi.org/10.1016/j.jhazmat.2011.03.027
Wang C, Liu Y, Hu H, Gao GX, Liu L, Chang SW, Guo JH, Chang L, Ouyang Y (2013) Electrochemical behavior of cerium ion in molten LiCl-KCl. J Rare Earths 31:405–409. https://doi.org/10.1016/S1002-0721(12)60295-6
Mendes E, Malmbeck R, Nourry C, Souček P, Glatz J-P (2012) On the electrochemical formation of Pu–Al alloys in molten LiCl–KCl. J Nucl Mater 420:424–429. https://doi.org/10.1016/j.jnucmat.2011.09.017
Khalaghi B, Kvalheim E, Tokushige M, Teng L, Seetharaman S, Haarberg GM (2014) Electrochemical behaviour of dissolved iron chloride in KCl+LiCl+NaCl Melt at 550ºC. ECS Trans 64:301–310. https://doi.org/10.1149/06404.0301ecst
Geran S, Chamelot P, Serp J, Gibilaro M, Massot L (2020) Electrochemistry of uranium in molten LiCl-LiF. Electrochim Acta 355:136784. https://doi.org/10.1016/j.electacta.2020.136784
Shirai O, Iwai T, Suzuki Y, Sakamura Y, Tanaka H (1998) Electrochemical behavior of actinide ions in LiCl–KCl eutectic melts. J Alloys Compd 271–273:685–688. https://doi.org/10.1016/S0925-8388(98)00187-X
Liu Z, Lu G, Yu J (2021) Investigation on electrochemical behaviors of MgCl2 impurity in LiCl-KCl melt. J Electrochem Soc 886:115131. https://doi.org/10.1016/j.jelechem.2021.115131
Serp J, Konings R, Malmbeck R, Rebizant J, Scheppler C (2004) Glatz J-P (2004) Electrochemical behaviour of Pu ion in LiCl–KCl eutectic melts. J Electrochem Soc 561:143–148. https://doi.org/10.1016/j.jelechem.2003.07.027
Yang Q, Ge J (2021) Zhang J (2021) Electrochemical study on the kinetic properties of Fe2+/Fe, Ni2+/Ni, Cr2+/Cr and Cr3+/Cr2+ in molten MgCl2-KCl-NaCl salts. J Electrochem Soc 168:012504. https://doi.org/10.1149/1945-7111/abdafc
Saïla A, Gibilaro M, Massot L, Chamelot P, Taxil P, Affoune A-M (2010) Electrochemical behaviour of dysprosium(III) in LiF–CaF2 on Mo, Ni and Cu electrodes. J Electroanl Chem 642:150–156. https://doi.org/10.1016/j.jelechem.2010.03.002
Osipenko A, Maershin A, Smolenski V, Novoselova A, Kormilitsyn M, Bychkov A (2011) Electrochemical behaviour of curium(III) ions in fused 3LiCl–2KCl eutectic. J Electrochem Soc 651:67–71. https://doi.org/10.1016/j.jelechem.2010.10.027
Osipenko A, Maershin A, Smolenski V, Novoselova A, Kormilitsyn M, Bychkov A (2010) Electrochemistry of oxygen-free curium compounds in fused NaCl–2CsCl eutectic. J Nucl Mater 396:102–106. https://doi.org/10.1016/j.jnucmat.2009.10.061
Yamada D, Murai T, Moritani K, Sasaki T, Takagi I, Moriyama H, Kinoshita K, Yamana H (2007) Diffusion behavior of actinide and lanthanide elements in molten salt for reductive extraction. J Alloys Compd 444–445:557–560. https://doi.org/10.1016/j.jallcom.2007.02.153
Shirai O, Iwai T, Shiozawa K, Suzuki Y, Sakamura Y, Inoue T (2000) Electrolysis of Pu nitride in LiCl–KCl eutectic melts. J Nucl Mater 277:226–230. https://doi.org/10.1016/S0022-3115(99)00194-4
Roy J, Grantham L, Grimmett D, Fusselman S, Krueger C, Storvick T, Inoue T, Sakamura Y, Takahashi N (1996) Thermodynamic properties of U, Np, Pu, and Am in molten LiCl-KCl eutectic and liquid cadmium. J Electrochem Soc 143:2487–2492. https://doi.org/10.1149/1.1837035
Masset P, Konings RJ, Malmbeck R, Serp J (2005) Glatz J-P (2005) Thermochemical properties of lanthanides (Ln =La, Nd) and actinides (An = U, Np, Pu, Am) in the molten LiCl–KCl eutectic. J Nucl Mater 344:173–179. https://doi.org/10.1016/j.jnucmat.2005.04.038
Marsden K, Pesic B (2011) Evaluation of the electrochemical behavior of CeCl3 in molten LiCl-KCl eutectic utilizing metallic Ce as an anode. J Electrochem Soc 158:F111–F120. https://doi.org/10.1149/1.3575637
Tang H, Pesic B (2014) Electrochemical behavior of LaCl3 and morphology of La deposit on molybdenum substrate in molten LiCl–KCl eutectic salt. Electrochim Acta 119:120–130. https://doi.org/10.1016/j.electacta.2013.11.148
Acknowledgements
We gratefully acknowledge the financial support provided by the National Natural Science Foundation of China (Grant No. U2167223).
Author information
Authors and Affiliations
Contributions
RL: Methodology, writing—original draft. ZM: Investigation, writing—original draft. YW: Data analysis. HC: Investigation. PS: Investigation. YJ: Data analysis YL: Writing—editing and review, supervision, resources. HH: Resources, methodology, writing − editing and review, supervision. GY: Conceptualization, writing—editing and review, supervision.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lin, R., Meng, Z., Wang, Y. et al. Electrochemical and thermodynamic properties of Pu(III) ions at the Mo electrode in LiCl–KCl eutectics. J Radioanal Nucl Chem 333, 23–30 (2024). https://doi.org/10.1007/s10967-023-09237-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-023-09237-0