Abstract
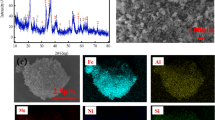
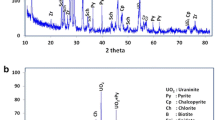
To investigate the leaching rules and mechanism of a hard rock uranium ore, this paper conducted conventional leaching experiments based on the chemical composition and mineral occurrence status of the ore. Various factors affecting leaching were explored, and experimental data were fitted using the shrinking-core model. The results showed that uranium in the ore mainly existed in the form of pitchblende and coffinite, and the uranium grade of the sample was 0.15%. Under the leaching conditions of a temperature of 50 °C, a particle size of − 100 mesh, an acid dosage of 32 kg t−1, a pyrolusite dosage of 10 kg t−1, a liquid–solid ratio of 1:1, and an 8 h duration, the leaching rate of the uranium ore reached 99.12%. The leaching process was controlled by solid film diffusion, and the apparent activation energy was 11.6 kJ mol−1. Insoluble substances were present on the surface of the slag, and the solid film product was the main reason hindering leaching.








Similar content being viewed by others
References
Tang R, Fan S (2022) The value concept, internal mechanism and path choice of the “double carbon” strategy to promote common prosperity. J Guizhou Norm Univ (Soc Sci Ed.) 6:78–90
Huang S, Wang JY, Guo P, Li ZN (2022) Short term strategy and long-term prospect of energy structure optimization under the carbon neutrality goal. Chem Ind Eng Prog 41(11):5695–5708
Ye GA, Zheng WF, He H, Ma J, Wang J (2020) Current status and development of nuclear fuel reprocessing technology in China. Atom Energ Sci Technol 54(S1):75–83
Luo XP, Chen JA, Xiong SH (2016) Recent progress in chemical beneficiation technology. Sichuan Nonferr Metals 3:13–19+12
Liu H, Meng YS, Zhang JM (2021) Experimental study on roasting of a siliceous mudstone uranium ore. Guangdong Chem Ind. 48(12):41–43
Han W, Liu H, Zhang H (2020) Experimental study on chlorination roasting of a volcanic rock type uranium polymetallic ore. Guangdong Chem Industry 47(12):53–55
Kang SH, Meng J, Wang HP, Wang P, Yang JF, Li DB, Fan X (2013) Study on the enhanced leaching process of a certain uranium molybdenum mine. Nonferr Metals (Extract Metall). 9:45–48+59
Cai XM (1990) Study on leaching of uranium ore with ferric sulfate. China Nucl Sci Technol Report 00:309–316
Li MT, Wei C, Zhou XJ et al (2012) Kinetics of vanadium leaching from black shale in non-oxidative conditions. Mineral Process Extract Metall 121(1):40–47
Makanyire T, Jha A, Sutcliffe S (2016) Kinetics of hydrochloric acid leaching of niobium from TiO2 residues. Int J Miner Process 157:1–6
Yang S, Hao LI, Sun Y et al (2016) Leaching kinetics of zinc silicate in ammonium chloride solution. Trans Nonferr Metals Soci China 26(6):1688–1695
Liu ZH, Cao ZY, Liu ZY (2012) Leaching kinetics of silicon zinc ore in a high liquid-solid ratio (NH4) 2SO4-NH3-H2O system. J Central South Univer (Sci Technol). 43(2):418–423
Ramos-Cano J, González-Zamarripa G, Carrillo-Pedroza FR et al (2016) Kinetics and statistical analysis of nickel leaching from spent catalyst in nitric acid solution. Int J Miner Process 148:41–47
Sinha S, Meshram P, Pandey BD (2016) Metallurgical processes for the recovery and recycling of lanthanum from various resources—a review. Hydrometallurgy 160:47–59
MacCarthy J, Nosrati A, Skinner W et al (2016) Atmospheric acid leaching mechanisms and kinetics and rheological studies of a low grade saprolitic nickel laterite ore. Hydrometallurgy 160:26–37
Zhou S, Chen B, Wang M et al (2016) Kinetics of extracting vanadium from stonecoal by alkali leaching. Rare Metal Technol 2016:159–165
Tanda BC, Eksteen JJ, Oraby EA (2018) Kinetics of chalcocite leaching in oxygenated alkaline glycine solutions. Hydrometallurgy 178:264–273
Zen T, Deng ZG, Zhang F (2020) Study on the leaching behavior and kinetics of copper in the process of zinc kiln slag leaching with waste acid. J Central South Univer (Sci Technol). 51(6):1489–1500
Li HR, Feng YL, Luo XB (2008) Kinetics of leaching vanadium from clay minerals. J Central South University (Sci Technol). 39(6):1181–1184
Shen J, Huang G, An C et al (2018) Removal of tetrabromobisphenol A by adsorption on pinecone-derived activated charcoals: synchrotron FTIR, kinetics and surface functionality analyses. Biores Technol 247:812–820
Behera SS, Parhi PK (2016) Leaching kinetics study of neodymium from the scrap magnet using acetic acid. Sep Purif Technol 160:59–66
Peng HR, Gong MM, Chen YZ et al (2017) Thermal stability of nanocrystalline materials: thermodynamics and kinetics. Int Mater Rev 62(6):303–333
Lin M, Liu YY, Lei SM et al (2018) High-efficiency extraction of Al from coal-series kaolinite and its kinetics by calcination and pressure acid leaching. Appl Clay Sci 161:215–224
Liu YL, Li GY, Wang YT, Hu N, Yu Q, Ma L, Ding DX (2018) Kinetic analysis of acidification bacteria column leaching of uranium ore. Atom Energ Sci Technol 52(2):227–234
Zheng Y, Chen K (2014) Leaching kinetics of selenium from selenium–tellurium-rich materials in sodium sulfite solutions. Trans Nonferr Metals Soci China 24(2):536–543
Ferrier RJ, Cai L, Lin Q et al (2016) Models for apparent reaction kinetics in heap leaching: a new semi-empirical approach and its comparison to shrinking core and other particle-scale models. Hydrometallurgy 166:22–33
Mishra B, Gubel N R, Bhola R (2013) Uranium processing and properties. Uranium processing. 123–172
Ho EM, Quan CH (2007) Iron (II) oxidation by SO2/O2 for use in uranium leaching. Hydrometallurgy 85(2–4):183–192
Acknowledgements
This project was major supported by the Nuclear energy development project (technology for the mining and metallurgy of associated uranium resources – on the demonstration of uranium co-mining in Bayan Ura, Inner Mongolia) and China Uranium Industry Co., Ltd.- the Foundation of State Key Laboratory of Nuclear Resources and Environment Joint Innovation Fund Project (2022NRE-LH-15) .
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, Z., Li, J., Li, H. et al. Research on conventional leaching process and leaching kinetics of a hard rock uranium mine. J Radioanal Nucl Chem 332, 4929–4942 (2023). https://doi.org/10.1007/s10967-023-09234-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-023-09234-3