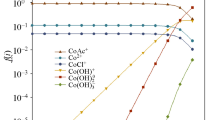
Abstract
Study of the influence of zeolite activation temperature on the sorption of a mixture of remove radionuclides Cs, Sr, Co. The activation temperature ranged from 150 to 450 °C. It was found that temperature activation did not lead to changes in clinoptilolite structure. After thermal activation, in all the considered temperature ranges, the sorption properties of clinoptilolite decreased by 15%. For composite sorbents, activation at 150 °C was the most effective. In this case, composite sorbents exhibited complex interactions with the mixture of radionuclides. Cs sorption decreased by 7%, Sr sorption increased by 1%, and Co sorption increased by 10–12%.


Similar content being viewed by others
References
Ding H, Wang YB, Liang N, Wang BK (2010) Activation of natural zeolite and its adsorption property. Adv Mater Res. https://doi.org/10.4028/www.scientific.net/AMR.178.3
Król MK, Jeleń P (2021) The effect of heat treatment on the structure of zeolite A. Materials (Basel). https://doi.org/10.3390/ma14164642
de Gennaro B, Cappi A, de Gennaro M, Bianco N, Langella A, Cappelletti P, Marocco A, Aprea P, Pansini M (2022) Use of Zeolites in the capture and storage of thermal energy by water desorption–adsorption cycles. Materials. https://doi.org/10.3390/ma15165574
Liu Z, Xu J, Xu M, Huang C, Wang R, Li T, Huai X (2022) Ultralow-temperature-driven water-based sorption refrigeration enabled by low-cost zeolite-like porous aluminophosphate. Nat Commun. https://doi.org/10.1038/s41467-021-27883-4
Cadar O, Senila M, Hoaghia MA, Scurtu D, Miu I, Levei EA (2020) Effects of thermal treatment on natural clinoptilolite-rich zeolite behavior in simulated biological fluids. Molecules. https://doi.org/10.3390/molecules25112570
Akkocaa DB, Yιlgιnb M, Urala M, Akçinc H, Mergend A (2013) Hydrothermal and thermal treatment of natural clinoptilolite zeolite from Bigadiç, Turkey: an experimental study. Geochem Int. https://doi.org/10.1134/S0016702913040022
Kukobat R, Škrbić R, Massiani P, Baghdad K, Launay F, Sarno M, Cirillo C, Senatore A, Salčin E, Atlagić SG (2022) Thermal and structural stability of microporous natural clinoptilolite zeolite. Microporous Mesoporous Mater. https://doi.org/10.1016/j.micromeso.2022.112101
Erdoğan B, Dikmen G, Alver Ö (2019) The analyses of the effect of heat treatment on the structural properties of zeolites from turkey using FT-IR, MAS NMR and XRD methods/Eskişehir technical university journal of science and technology. A Appl Sci Eng 5:54–668. https://doi.org/10.18038/estubtda.519641
Bayrakdar Ates E (2022) Investigating the chemical and thermal based treatment procedures on the clinoptilolite to improve the physicochemical properties. Bayrakdar Ates E JOTCSB 5(2):39–58
Breck D (1974) Zeolite molecular sieves. Wiley, New York
Cortés-Martínez R, Olguín MT, Solache-Ríos M (2010) Cesium sorption by clinoptilolite-rich tuffs in batch and fixed-bed systems. Desalination. https://doi.org/10.1016/j.desal.2010.03.019
Prajitno MY, Taufiqurrakhman M, Harbottle D, Hunter TN (2021) Kinetic studies of Cs+ and Sr2+ ion exchange using clinoptilolite in static columns and an agitated tubular reactor (ATR). ChemEngineering. https://doi.org/10.3390/chemengineering5010009
Lonin AYu, Levenets VV, Neklyudov IM, Shchur AO (2015) The usage of zeolites for dynamic sorption of cesium from waste waters of nuclear power plants. J Radioanal Nucl Chem DOI. https://doi.org/10.1007/s10967-014-3597-9
Levenets VV, Lonin AYu, Omelnik OP, Shchur AO (2016) Comparison the sorption properties of clinoptilolite and synthetic zeolite during sorption strontium from the water solutions in static conditions: sorption and quantitative determination of strontium by the method PIXE. J Environ Chem Eng. https://doi.org/10.1016/j.jece.2016.09.011
Lonin AYu, Levenets VV, Omelnik OP, Shchur AO (2018) Comparison of the sorption properties of natural and synthetic zeolites for the purification of aqueous solutions from cobalt: sorption of the cobalt from aqueous solutions in dynamic conditions and the quantitative determination of cobalt by the PIXE method. J Radioanal Nucl Chem DOI. https://doi.org/10.1007/s10967-017-5676-1
Rudenko LI, Dzhuzha OV, Khan VE (2007) Transuranic elements in liquid radioactive waste from the “Shelter” facility. Rep Natl Acad Sci Ukr 10:142–146
Lonin AYu, Кrasnopyorova AP (2005) Influence of different factors on sorption of 90Sr by natural and synthetic zeolites. Probl Atomic Sci Technol 6(45):130–132
Karpus SG, Kuzmenko VV, Levenets VV et al (2023) Modernization of the analytical nuclear-physical complex Sokil. Probl Atomic Sci Technol 2(144):134–139
Lonin AYu, Levenets VV, Omelnik OP, Shchur AO (2021) Use of sorbents composition (clinoptilollite and synthetic zeolite) for elimination of cesium and cobalt from aqueous solutions. J Radioanal Nucl Chem. https://doi.org/10.1007/s10967-021-07762-4
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lonin, O.Y., Levenets, V.V., Omelnik, O.P. et al. Sorption of Cs, Sr, and Co from a solution by a composite sorbent (ClSZ) based on zeolite depending on the activation temperature. J Radioanal Nucl Chem 332, 5087–5093 (2023). https://doi.org/10.1007/s10967-023-09224-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-023-09224-5