Abstract
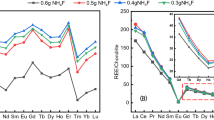
In order to analyze trace rare earth impurities in ThF4, two effective analytical methods based on the matrix-matched strategy using ICP-MS with different sample pretreatment procedures are developed in this study. The first procedure involves the conversion of ThF4 into ThO2 by pyrohydrolysis and the subsequent dissolution of ThO2 in 3M HNO3. In the second procedure, ThF4 is directly dissolved into hot ammonium carbonate solution. The detection limits, precisions, and recovery rates of both methods were investigated. For the 15 rare earth elements (REEs), the detection limits of the methods are in the range of 0.001–0.077 µg g−1. The recoveries of the rare earth impurities with concentrations of 0.05–50 µg g−1 ranged mostly from 96 to 110%, and the relative standard deviations have been determined to be less than 8%. Both analytical methods are composed of simple and green processes without generation of organic waste.

Similar content being viewed by others
Change history
02 January 2024
A Correction to this paper has been published: https://doi.org/10.1007/s10967-023-09324-2
References
Jiang MH, Xu HJ, Dai ZM (2012) Future advanced nuclear fission energy–TMSR nuclear energy system. Bull Chin Acad Sci 27:366–374
Nuttin A, Heuer D, Billebaud A, Brissot R, Le Brun C, Liatard E, Loiseaux JM, Mathieu L, Meplan O (2005) Potential of thorium molten salt reactors detailed calculations and concept evolution with a view to large scale energy production. Prog Nucl Energy 46:77–99
Li GC, Zou Y, Yu CG, Han JL, Chen JG, Xu HJ (2017) Influences of 7Li enrichment on Th–U fuel breeding for an improved molten salt fast reactor (IMSFR). Nucl Sci Tech 28:1–9
Kolay S, Phapale S, Dawar R, Jafar M, Achary SN, Tyagi AK, Mishra R, Basu M (2017) Phase characterization and thermal study of SrF2 and NdF3 doped fluoride salt containing 78 mol% LiF-20 mol% ThF4-2 mol% UF4. J Radioanal Nucl Ch 314:1267–1271
Bürger S, Boulyga SF, Peńkin MV, Bostick D, Jovanovic S, Lindvall R, Rasmussen G, Riciputi L (2014) Quantifying multiple trace elements in uranium ore concentrates: an interlaboratory comparison. J Radioanal Nucl Ch 301:711–729
Kayasth SR, Desai HB, Das MS (1985) Determination of traces of rare earths in high–purity thorium dioxide by neutron activation analysis. Anal Chim Acta 187:271–277
Gopalkrishnan M, Radhakrishnan K, Dhami PS, Kulkarni VT, Mathur JN (1997) Determination of trace impurities in uranium, thorium and plutonium matrices by solvent extraction and inductively coupled plasma atomic emission spectrometry. Talanta 44:169–176
Edge RA (1963) A combined anion exchange-solvent extraction procedure for separating trace amounts of Eu, Gd, Dy, Sm and Er from thorium tetrafluoride. Anal Chim Acta 28:278–281
Okumura I, Shimada S, Higashi K (1973) Spectrophotometric determination of uranium in thorium tetrafluoride. Anal Chem 45:1945–1945
Kelkar A, Prakash A, Afzal M, Panakkal JP, Kamath HS (2010) Determination of alkali, alkaline earth and transition metal ions in UO2, ThO2 powders and sintered (Th, U)O2 pellets by ion chromatography. J Radioanal Nucl Ch 284:443–449
Alamelu D, Choudhary AK, Aggarwal SK (2010) Determination of impurities in thoria (ThO2) using laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS). J Nucl Mater 406:356–359
Kayasth SR, Desai HB, Das MS (1985) Determination of traces of rare earths in high-purity thorium dioxide by neutron activation analysis. Anal Chim Acta 187:271–277
Kulkarni MJ, Argekar AA, Mathur JN, Page AG (1998) Chemical separation and inductively coupled plasma–atomic emission spectrometric determination of seventeen trace metals in thorium oxide matrix using a novel extractant-Cyanex-923. Anal Chim Acta 370:163–171
Nelms JR, Vogel RS (1966) Direct spectrometric determination of Dy, Eu, Gd, and Sm in high–purity thorium dioxide. Appl Spectrosc 21:242–245
Porwal NK, Argekar AA, Purohit PJ, Page AG, Sastry MD (1990) Trace metal assay of thorium oxide by atomic emission spectrometry. Fresen J Anal Chem 338:255–258
Langmuir D, Herman JS (1980) The mobility of thorium in natural waters at low temperatures. Geochim Cosmochim Ac 44:1753–1766
Smith FJ, Mesmer RE, Mctaggart DR (1981) The solubility of thorium fluoride in nitric acid-hydrofluoric acid solution between 25 and 100 °C. J Inorg Nucl Chem 43:541–547
Nikolaev NS, Luk’Yanychev YA (1962) The determination of the solubility product of thorium tetrafluoride. Sov Atomic Energy 12:357–358
Wickleder MS, Fourest B, Dorhout PK (2006) The chemistry of the actinide and transactinide elements, 3rd edn. Springer, Berlin
Östhols E (1995) Thorium sorption on amorphous silica. Geochim Cosmochim Acta 59:1235–1249
Sthols E, Bruno J, Grenthe I (1994) On the influence of carbonate on mineral dissolution: III. The solubility of microcrystalline ThO2 in CO2–H2O media. Geochim Cosmochim Acta 58:613–623
Dong XY, Zheng XB, Song YL, Liu YX, Zhang L (2014) Pyrohydrolysis of uranium tetrafluoride and thorium tetrafluoride. J Nucl Radiochem 36:181–185 (in Chinese)
ASTM C1502-16 (2016) Standard test method for determination of total chlorine and fluorine in uranium dioxide and gadolinium oxide
Tian M, Han XY, Wang J, Zhang RR (2012) Study on correction of matrix effect with different internal standards during measurement of uranium by ICP–MS. Chin J Anal Lab 31:116–120 (in Chinese)
HJ Ministry of environmental protection of PRC (2010) Environmental monitoring-technical guideline on drawing and revising analytical method standards. HJ 168–2010
Acknowledgements
This study was supported by the Frontier Science Key Program of the Chinese Academy of Sciences (Grant Number QYZDY-SSW-JSC016).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original article has been revised due to the interchange the given and family name of the author as “Yuan Qian instead of Qian Yuan”
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Han, L., Li, X., Cao, C. et al. Effective determination of trace rare earth elements in ThF4 by ICP-MS with different material pretreatment procedures. J Radioanal Nucl Chem 333, 451–457 (2024). https://doi.org/10.1007/s10967-023-09202-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-023-09202-x