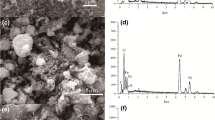
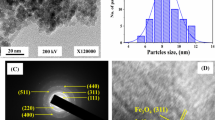
Abstract
Modified chitosan-coated nickel ferrite silica nanoparticles were prepared, characterized and experimented for the removal of U(VI). Adsorption amount was 125.30 mg g−1 at pH 5.0. The magnetic chitosan based composite could be successfully recycled from uranium aqueous phase. Finally, calculated data and analysis suggested that the adsorption behaviors of magnetic chitosan based nanoparticles can be explained by pseudo-second order kinetic model (R2 = 0.9915), which suggested the adsorption process was probably controlled by chemical adsorption, and langmuir model (R2 = 0.9816), which suggested the adsorption process was probably controlled by single-layer adsorption.








Similar content being viewed by others
References
Ashrafi F, Firouzzare M, Ahmadi SJ, Sohrabi MR, Khosravi M (2019) Preparation and modification of forcespun polypropylene nanofibers for adsorption of uranium(VI) from simulated seawater. Ecotoxicol Environ Saf 186:109746
Yuan Y, Yu Q, Cao M, Feng L, Feng S, Liu T, Feng T, Yan B, Guo Z, Wang N (2021) Selective extraction of uranium from seawater with biofouling-resistant polymeric peptide. Nat Sustain 4:708–714
Li N, Wu J, Su R, Zhang N, Zhao J, Wang Z (2023) Bioinspired green tea waste/ graphene aerogel for solar-enhanced uranium extraction from seawater. Desalination 545:116153
Xu M, Zhou L, Zhang L, Zhang S, Chen F, Zhou R, Hua D (2022) Two-dimensional imprinting strategy to create specific nanotrap for selective uranium adsorption with ultrahigh capacity. ACS Appl Mater Interfaces 14:9408–9417
Dewar D (2019) Uranium mining: environmental and human health effects. In Nuclear non-proliferation in international law-volume IV TMC Asser Press. The Hague pp:229–235
Dinis MD, Fiúza A (2021) Mitigation of uranium mining impacts—a review on groundwater remediation technologies. Geosciences 6:250
Bangotra P, Sharma M, Mehra R, Jakhu R, Singh A, Gautam AS, Gautam S (2021) A systematic study of uranium retention in human organs and quantification of radiological and chemical doses from uranium ingestion. Environ Technol Innov 21:101360
Yu J, Wang J, Zhu J, Li Y, Liu Q, Yu J, Li R, Liu P, Zhang H (2023) Interaction mechanism of uranium(VI) with chitosan hydrogel: insights from the perspective of adsorbent and adsorbate. Desalination 546:116194
Hong J, Ma R, Wu Y, Liu Y, Wen T, Zhang S, Wang S, Wang X, Ai Y (2022) Experimental and theoretical identifications of durable Fe-Nx configurations embedded in graphitic carbon nitride for uranium photoreduction. J Environ Chem Eng 10:108374
Wang R, Li M, Liu T, Li X, Zhou L, Tang L, Gong C, Gong X, Yu K, Li N, Zhu W, Chen T (2022) Encapsulating carbon-coated nano zero-valent iron particles with biomass-derived carbon aerogel for efficient uranium extraction from uranium-containing wastewater. J Clean Prod 364:132654
Huang S, Pang H, Li L, Jiang S, Wen T, Zhuang L, Hu B, Wang X (2018) Unexpected ultrafast and high adsorption of U(VI) and Eu(III) from solution using porous Al2O3 microspheres derived from MIL-53. Chem Eng J 353:157–166
Wang G, Zhen J, Zhou L, Wu F, Deng N (2015) Adsorption and photocatalytic reduction of U(VI) in aqueous TiO2 suspensions enhanced with sodium formate. J Radioanal Nucl Chem 304:579–585
Ma S, Zhan S, Jia Y, Zhou Q (2015) Highly efficient antibacterial and Pb(II) removal effects of Ag-CoFe2O4-GO nanocomposite. ACS Appl Mater Interfaces 7:10576–10586
Zhang S, Niu H, Cai Y, Zhao X, Shi Y (2010) Arsenite and arsenate adsorption on coprecipitated bimetal oxide magnetic nanomaterials: MnFe2O4 and CoFe2O4. Chem Eng J 158:599–607
Song Q, Zhang ZJ (2012) Controlled synthesis and magnetic properties of bimagnetic spinel ferrite CoFe2O4 and MnFe2O4 nanocrystals with core-shell architecture. J Am Chem Soc 134:10182–10190
Dai Z, Zhen Y, Sun Y, LiDing LD (2021) ZnFe2O4/g-C3N4 S-scheme photocatalyst with enhanced adsorption and photocatalytic activity for uranium(VI) removal. Chem Eng J 415:129002
Lingamdinne L, Choi Y, Kim I, Yang J, Koduru J, Chang Y (2017) Preparation and characterization of porous reduced graphene oxide based inverse spinel nickel ferrite nanocomposite for adsorption removal of radionuclides. J Hazard Mater 326:145–156
Burillo JC, Ballinas L, Burillo G, Guerrero-Lestarjette E, Lardizabal-Gutierrez D, Silva-Hidalgo H (2021) Chitosan hydrogel synthesis to remove arsenic and fluoride ions from groundwater. J Hazard Mater 417:126070
Liu L, Yang W, Gu D, Zhao X, Pan Q (2019) In situ preparation of chitosan/ZIF-8 composite beads for highly efficient removal of U(VI). Front Chem 7:607
Wang G, Liu J, Wang X, Xie Z, Deng N (2009) Adsorption of uranium (VI) from aqueous solution onto cross-linked chitosan. J Hazard Mater 168:1053–1058.
Guo X, Yang H, Liu Q, Liu J, Chen R, Zhang H, Yu J, Zhang M, Li R, Wang J (2020) A chitosan-graphene oxide/ZIF foam with anti-biofouling ability for uranium recovery from seawater. Chem Eng J 382:122850
Ganji P, Nazari S, Zinatizadeh AA, Zinadini S (2022) Chitosan-wrapped multiwalled carbon nanotubes (CS/MWCNT) as nanofillers incorporated into nanofiltration (NF) membranes aiming at remarkable water purification. J Water Process Eng 48:102922
Zhuang ST, Yin YN, Wang JL (2017) Removal of cobalt ions from aqueous solution using chitosan grafted with maleic acid by gamma radiation. Nucl Eng Technol 50:211–215
Cai YW, Chen L, Yang ST, Xu L, Qin HB, Liu ZY, Chen LH, Wang XK, Wang S (2019) Rational synthesis of novel phosphorylated chitosancarboxymethyl cellulose composite for highly effective decontamination of U(VI). ACS Sustain Chem Eng 7:5393–5403
Bin Xu Z, Wang WL, Huang N, Wu QY, Lee MY, Hu HY (2019) 2-Phosphonobutane-1,2,4-tricarboxylic acid (PBTCA) degradation by ozonation: Kinetics, phosphorus transformation, anti-precipitation property changes and phosphorus removal. Water Res 148:334–343
Gou SH, Zhou YT, Duan M, Peng C, Yang XY, Wang J (2018) Amidoximemodified chitosan for pigment red 224 enrichment through reversible assembly. New J Chem 42:1492–1500
Jiang XY, Wang HQ, Wang QL, Hu EM, Duan YQ (2020) Immobilizing aminofunctionalized mesoporous silica into sodium alginate for efficiently removing low concentrations of uranium. J Clean Prod 247:119162
Jiang XY, Wang HQ, Hu EM, Lei ZW, Wang QL (2020) Efficient adsorption of uranium from aqueous solutions by microalgae based aerogel. Microporous Mesoporous Mater 305:110383
Pan M, Sun Y, Zheng J, Yang W (2013) Boronic acid-functionalized core-shell-shell magnetic composite microspheres for the selective enrichment of glycoprotein. ACS Appl Mater Interfaces 5:8351–8358
Huang Y, Wu H, Shao T, Zhao X, Peng H, Gong Y, Wan H (2018) Enhanced copper adsorption by DTPA-chitosan/alginate composite beads: Mechanism and application in simulated electroplating wastewater. Chem Eng J 339:322–333
Li X, Liu X, Lin C, Zhang H, Zhou Z, Fan G, Ma J (2019) Cobalt ferrite nanoparticles supported on drinking water treatment residuals: an efficient magnetic heterogeneous catalyst to activate peroxymonosulfate for the degradation of atrazine. Chem Eng J 367:208–218
Deng J, Shao Y, Gao N, Tan C, Zhou S, Hu X (2013) CoFe2O4 magnetic nanoparticles as a highly active heterogeneous catalyst of oxone for the degradation of diclofenac in water. J Hazard Mater 262:836–844
Habiba U, Afifi AM, Salleh A, Ang BC (2017) Chitosan/(polyvinyl alcohol)/zeolite electrospun composite nanofibrous membrane for adsorption of Cr6+, Fe3+ and Ni2+. J Hazard Mater 322:182–194
Sutirman ZA, Sanagi MM, Abd Karim KJ, Wan Ibrahim WA (2016) Preparation of methacrylamide-functionalized crosslinked chitosan by free radical polymerization for the removal of lead ions. Carbohydr Polym 151:1091–1099
Ren Y, Abbood HA, He F, Peng H, Huang K (2013) Magnetic EDTA-modified chitosan/SiO2/Fe3O4 adsorbent: preparation, characterization, and application in heavy metal adsorption. Chem Eng J 226:300–311
Zeng J, He B, Lamb K, Marco RD, Shen PK, Jiang SP (2013) Phosphoric acid functionalized pre-sintered meso-silica for high temperature proton exchange membrane fuel cells. Chem Commun 49:4655–4657
Zou CJ, Tang QW, Lan GH, Tian Q, Wang TY (2013) Enhancement inhibition efficiency of PBTCA depending on the inclusion complex with hydroxypropyl-β-cyclodextrin. J Incl Phenom Macrocycl Chem 76:61–68
Fang Y, Yuan X, Wu L, Peng Z, Feng W, Liu N, Xu D, Li S, Sengupta A, Mohapatra PK, Yuan L (2015) Ditopic CMPO-pillar[5]arenes as unique receptors for efficient separation of americium(iii) and europium(iii). Chem Commun 51:4263–4266
He F, Lu Z, Song M, Liu X, Tang H, Huo P, Fan W, Dong H, Wu X, Han S (2019) Selective reduction of Cu2+ with simultaneous degradation of tetracycline by the dual channels ion imprinted POPD-CoFe2O4 heterojunction photocatalyst. Chem Eng J 360:750–761
Lingamdinne LP, Choi JS, Angaru GKR, Karri RR, Yang JK, Chang YY, Koduru JR (2022) Magnetic-watermelon rinds biochar for uranium-contaminated water treatment using an electromagnetic semi-batch column with removal mechanistic investigations. Chemosphere 286:131776
Zhou S, Xie Y, Zhu F, Gao Y, Liu Y, Tang YD (2021) Amidoxime modified chitosan/graphene oxide composite for efficient adsorption of U(VI) from aqueous solutions. J Environ Chem Eng 9:106363
Lei H, Pan N, Wang X, Zou H (2018) Facile synthesis of phytic acid impregnated polyaniline for enhanced U(VI) adsorption. J Chem Eng Data 63:3989–3997
Zhang M, Li Y, Bai C, Guo X, Han J, Hu S, Jiang H, Tan W, Li S, Ma L (2018) Synthesis of microporous covalent phosphazene-based frameworks for selective separation of uranium in highly acidic media based on size-matching effect. ACS Appl Mater Interfaces 10:28936–28947
Hosseini M, Keshtkar AR, Moosavian MA (2016) Electrospun chitosan/baker’s yeast nanofibre adsorbent: preparation, characterization and application in heavy metal adsorption. Bull Mater Sci 39:1091–1100
Khan A, Xing J, Elseman AM, Gu P, Gul K, Ai Y, Jehan R, Alsaedi A, Hayat T, Wang X (2018) A novel magnetite nanorod-decorated Si-Schiff base complex for efficient immobilization of U (VI) and Pb (II) from water solutions. Dalton Trans 47:11327–11336
Wang T, Xu M, Han X, Yang S, Hua D (2019) Petroleum pitch-based porousaromatic frameworks with phosphonate ligand for efficient separation of uranium from radioactive effluents. J Hazard Mater 368:214–220
Sun Y, Lan J, Li M, Hu W, Liu H, Song G, Chen D, Shi W, Wang X (2018) Influence of aqueous sulfide on speciation of U(vi) adsorbed to nanomagnetite. Environ Sci Nano 5:1981–1989
Acknowledgements
The study was financially supported by the National Natural Science Foundation of China (22006012), Engineering Research Center of Nuclear Technology Application (East China University of Technology) (HJSJYB2021-15)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, W., Wang, Z. Magnetic NiFe2O4@SiO2@CS-PBTCA nanoparticles for uranium adsorption. J Radioanal Nucl Chem 332, 5007–5016 (2023). https://doi.org/10.1007/s10967-023-09193-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-023-09193-9