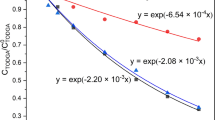
Abstract
Third phase formation during nuclear solvent extraction has the delirious effect on operation of extractor cascade and may result in the criticality due to unsafe accumulation of the actinides. In this study, the nitric acid third phase was generated with 30%TBP-dodecane/nitric acid system and characterization studies were performed. The earlier model of author and coworker for U(IV) and Pu(IV) third phase could be extended to cover the current study. Experimental results indicated a presence of chain-linked nitric acid dimer to a single molecule of TBP in the generated nitric acid third phase whereas speciation in the light phase was similar to unpartitioned organic phase.





Similar content being viewed by others
References
Clark AE (2019) Amphiphile- based complex fluids: the self assembly ensemble as protagonist. ACS Cen Sci 6:10–12
Servis MJ, Wu DT, Shafer JC, Clark AE. Preprint archive https://chemrxiv.org/engage/api-gateway/chemrxiv/assets/orp/resource/item/60c745954c8919daabad2a01/original/reimagining-third-phase-formation-as-the-miscibility-gap-of-a-molecular-solution.pdf. Accessed 12 May 2023
Servis MJ, Stephenson, Mesostructuring in liquid-liquid extraction. Organic phase originating from critical points. https://www.osti.gov/servlets/purl/1831745. Accessed 12 May 2023
Kumari I, Kumar BVR, Khanna A (2020) A review on UREX processes for nuclear spent fuel reprocessing. Nucl Eng Des 358:110410. https://doi.org/10.1016/j/nucengdes.2019.110410
Taylor R, Carrot M, Galan H, Geist A, Heres X, Maher C, Mason C, Malmbeck R, Miguirditchian M, Modolo G, Rhodes C, Sarsfield M, Wilden A (2016) The EURO-GANEX process: current status of flowsheet development and process safety studies. Proc Chem 21:524–529
Authen TL, Wilden A, Schneider D, Kreft F, Modolo G, Foreman MRS, Ekberg C (2021) Batch flowsheet test for a GANEX-type process: the CHALMEX FS-13 Process. Solv Extr Ion Exch. https://doi.org/10.1080/07366299.2021.1890372
Burger LL, Slansky CM (1949) Density and viscosity of solutions in the tributyl phosphate process for uranium recovery. Technical Report HW-15233, Hanford Work USA
Alcock K, Grimley SS, Healy TV, Kennedy J, McKay HAC (1956) The extraction of nitrates by tri-n-butyl phosphate (TBP), Part 1—the system TBP+diluent+H2O+HNO3. Trans Faraday Soc 52:39–47
Healy TV, McKay HAC (1956) The extraction of nitrates by tri-n-butyl phosphate (TBP), Part 2—the nature of TBP phase. Trans Faraday Soc 52:633–642
Mills AL, Logan WR (1967) In: Solvent extraction chemistry, Proc Intl Conf Gothenburg, Sweden. Dyrssen D, Liljenjin JO, Rydberg I (Eds.) North Holland, Amsterdam
Horner DE (1971) Formation of third phases and the effect of temperature on the distribution of plutonium and uranium in extractions by tri-n-butyl phosphate. Technical Report ORNL-4724, Oak Ridge National Laboratory, USA
Kolarik Z (1979) The formation of a third phase in the extraction of Pu(IV), U(IV) and Th(IV) nitrates with tributyl phosphate in alkane diluents. Proc Int Solv Extr Conf (ISEC-1977) CIM Spec 21:178–182
Gonda K, Oka K (1984) Accumulation process of plutonium third phase in mixer-settlers. Nucl Technol 64:14–18
Tachimori S, Suzuki S, Ami N (1993) Study of the third phase in U(IV)-HNO3 and tri-n-butyl phosphate-dodecane extraction system, Proc Int Solv Extr Conf (ISEC-93), York. Logsdail DH, Slater MJ (Eds) SCI & Elsevier: London, pp 1546–1553
Boukis N, Kanellakopulos B (1983) Extractive phase distribution of uranium nitrate with tri-n-butyl phosphate. Technical Report KfK-3352, Kernforschungszentrum, Karlsruhe
Kumar S, Koganti SB (1996) Empirical modeling of Pu(IV) third phase formation in 30%TBP/n-dodecane system. J Nucl Sci Technol 33:1003–1005
Kumar S, Koganti SB (2003) Speciation studies in third phase formation: U(IV), Pu(IV) and Th(IV) third phases in TBP systems. Solv Extr Ion Exch 21:547–558
Chiarizia R, Jensen MP, Borkowski M, Ferraro JR, Thiagarajan P, Littrell KC (2003) Third phase formation revisited: the U(VI), HNO3-TBP, n-dodecane system. Solv Extr Ion Exch 19:981–992
Plaue J, Gelis A, Czerwinski K, Thiagarajan P, Chiarizia R (2006) Small-angle neutron scattering study of plutonium third phase formation in 30%TBP/HNO3/alkane diluent system. Solv Extr Ion Exch 24:283–298
Plaue J, Gelis A, Czerwinski K (2006) Actinide third phase formation in 1.1M TBP/nitric acid/alkane diluent system. Sep Sci Technol 41:2065–2074
Ivanov P, Mu J, Leay L, Chang SY, Sharrad CA, Masters AJ, Schroeder SLM (2017) Organic and third phase in HNO3/TBP/n-dodecane system: no reverse Micelles. Solv Extr Ion Exch 34:251–265
Mu J (2017) Computer simulation study of third phase formation in a nuclear extraction process. PhD Thesis, University of Manchester UK
ASTM Standard D4052-11 (2011) Standard test method for density, relative density, and API gravity of liquids by digital density meter. ASTM International, West Conshohocken, PA, DOI: https://doi.org/10.1520/D4052-11
Siddall TH III, Parker SG, Prout WE (1957) Equilibrium Distribution data for PUREX and similar extraction processes. Technical Report DP-53, E I du Pont de Nemours & Co USA
Burger LL (1984) In: Chapter 3 of Science and Technology of Tributyl Phosphate, Vol I, Schulz WW, Navratil JD, Talbot AE (Eds.), CRC Press, Boca Raton, USA, p 25
Schaekers JM (1986) Estimation of equilibrium constants for the extraction of nitric acid by TBP/kerosene mixtures. Proc Int Solv Extr Conf (ISEC-1986), Munich, pp 185–192
Ferraro JR, Borkowski M, Chiarazia R, McAlister DR (2001) FT-IR spectroscopy of nitric acid in TBP/octane solution. Solv Extr Ion Exch 19:981–992
Ami N, Suzuki S, Abe H, Tachimori S (1993) Formation characteristics and its numerical models of the third phase in the U(IV)-HNO3-TBP-n-Dodecane System. Technical Report JAERI-M 93-014, JAERI, Tokai-Mura Japan
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
Author declares no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kumar, S. Speciation of nitric acid third phase in a 30%TBP/n-dodecane solvent. J Radioanal Nucl Chem 332, 4541–4548 (2023). https://doi.org/10.1007/s10967-023-09155-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-023-09155-1