Abstract
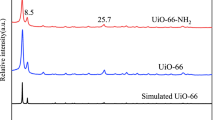
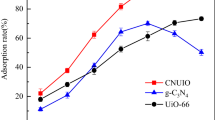
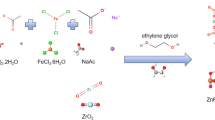
In this study, hydroxyl and carboxyl group functionalized Zr-based MOFs UiO-66 derivatives (UiO-66-R: UiO-66-(OH)2 and UiO-66-COOH) were prepared by a facile one-step method and used to remove Co(II) from simulated radioactive wastewater. The results showed that UiO-66-R have good adsorption properties with maximum adsorption capacities of 133.3 mg L−1 (UiO-66-(OH)2) and 125.6 mg L−1 (UiO-66-COOH). Furthermore, the Co(II) adsorption conforms to the pseudo-second-order kinetic model and Langmuir model, belonging to the single molecular layer chemisorption, and the adsorption processes are spontaneous and endothermic. XPS patterns illustrate that the coordination effect is crucial in determining the adsorption process of the two materials. To explore the irradiation stability, FT-IR, XRD and SEM were used to study the morphology and structure of materials after irradiation (20-80 kGy). The characterization analysis of UiO-66-R illustrated that UiO-66-(OH)2 and UiO-66-COOH showed little change before and after irradiation, which indicated that both materials have good irradiation stability.









Similar content being viewed by others
References
Liu Y, Serrano A, Vaughan J, Southam G, Zhao L, Villa-Gomez D (2019) The influence of biologically produced sulfide-containing solutions on nickel and cobalt precipitation reactions and particle settling properties. Hydrometallurgy. https://doi.org/10.1016/j.hydromet.2019.105142
Tang J, Sun H, Ma W, Feng M, Huang X (2020) Recent progress in developing crystalline ion exchange materials for the removal of radioactive ions. Chin J Struct Chem 39:2157–2171. https://doi.org/10.14102/j.cnki.0254-5861.2011-3018
Wang LY, Lee MS (2017) Separation of Co(II) and Ni(II) from chloride leach solution of nickel laterite ore by solvent extraction with Cyanex 301. Int J Miner Process 166:45–52. https://doi.org/10.1016/j.minpro.2017.07.004
Dambies L, Jaworska A, Zakrzewska-Trznadel G, Sartowska B (2010) Comparison of acidic polymers for the removal of cobalt from water solutions by polymer assisted ultrafiltration. J Hazard Mater 178:988–993. https://doi.org/10.1016/j.jhazmat.2010.02.035
Zhang M, Gu P, Yan S, Liu Y, Zhang G (2021) Effective removal of radioactive cobalt from aqueous solution by a layered metal sulfide adsorbent: mechanism, adsorption performance, and practical application. Sep Purif Technol. https://doi.org/10.1016/j.seppur.2020.117775
Adigun OA, Oninla VO, Babarinde NAA, Oyedotun KO, Manyala N (2020) Characterization of sugarcane leaf-biomass and investigation of its efficiency in removing nickel(II) chromium(III) and cobalt(II) ions from polluted water. Surf Interfaces. https://doi.org/10.1016/j.surfin.2020.100621
Adibmehr Z, Faghihian H (2019) Preparation of highly selective magnetic cobalt ion-imprinted polymer based on functionalized SBA-15 for removal Co2+ from aqueous solutions. J Environ Health Sci Eng 17:1213–1225. https://doi.org/10.1007/s40201-019-00439-x
Vivas EL, Cho K (2021) Efficient adsorptive removal of Cobalt(II) ions from water by dicalcium phosphate dihydrate. J Environ Manag 283:111990. https://doi.org/10.1016/j.jenvman.2021.111990
Siddiqui MN, Chanbasha B, Al-Arfaj AA, Kon’kova T, Ali I (2021) Super-fast removal of cobalt metal ions in water using inexpensive mesoporous carbon obtained from industrial waste material. Environ Technol Innov. https://doi.org/10.1016/j.eti.2020.101257
Long JR, Yaghi OM (2009) The pervasive chemistry of metal-organic frameworks. Chem Soc Rev 38:1213–1214. https://doi.org/10.1039/b903811f
Zhang Y, Wang L, Hu J, Duttwyler S, Cui X, Xing H (2020) Solvent-dependent supramolecular self-assembly of boron cage pillared metal–organic frameworks for selective gas separation. CrystEngComm 22:2649–2655. https://doi.org/10.1039/d0ce00142b
Dhainaut J, Bonneau M, Ueoka R, Kanamori K, Furukawa S (2020) Formulation of metal-organic framework inks for the 3D printing of robust microporous solids toward high-pressure gas storage and separation. ACS Appl Mater Interfaces 12:10983–10992. https://doi.org/10.1021/acsami.9b22257
Bauer G, Ongari D, Tiana D, Gäumann P, Rohrbach T, Pareras G, Tarik M, Smit B, Ranocchiari M (2020) Metal-organic frameworks as kinetic modulators for branched selectivity in hydroformylation. Nat Commun. https://doi.org/10.1038/s41467-020-14828-6
Lv S-W, Liu J-M, Li C-Y, Zhao N, Wang Z-H, Wang S (2019) A novel and universal metal-organic frameworks sensing platform for selective detection and efficient removal of heavy metal ions. Chem Eng J. https://doi.org/10.1016/j.cej.2019.122111
Winterlich M, McHugh D, O’Toole E, Skordi K, O’Malley C, Sanii R, Tasiopoulos A, Erxleben A, Mayans J, Morrison L, McArdle P, Zaworotko MJ, Tylianakis E, Froudakis G, Papatriantafyllopoulou C (2021) Expanding the NUIG MOF family: synthesis and characterization of new MOFs for selective CO2 adsorption, metal ion removal from aqueous systems, and drug delivery applications. Dalton Trans 50:6997–7006. https://doi.org/10.1039/d1dt00940k
Shahriyari Far H, Hasanzadeh M, Najafi M, Masale Nezhad TR, Rabbani M (2021) Efficient removal of Pb(II) and Co(II) ions from aqueous solution with a chromium-based metal-organic framework/activated carbon composites. Ind Eng Chem Res 60:4332–4341. https://doi.org/10.1021/acs.iecr.0c06199
Kobielska PA, Howarth AJ, Farha OK, Nayak S (2018) Metal–organic frameworks for heavy metal removal from water. Coord Chem Rev 358:92–107. https://doi.org/10.1016/j.ccr.2017.12.010
Abdollahi N, Akbar Razavi SA, Morsali A, Hu ML (2020) High capacity Hg(II) and Pb(II) removal using MOF-based nanocomposite: Cooperative effects of pore functionalization and surface-charge modulation. J Hazard Mater 387:121667. https://doi.org/10.1016/j.jhazmat.2019.121667
Su S, Che R, Liu Q, Liu J, Zhang H, Li R, Jing X, Wang J (2018) Zeolitic imidazolate framework-67: a promising candidate for recovery of uranium (VI) from seawater. Colloids Surf A 547:73–80. https://doi.org/10.1016/j.colsurfa.2018.03.042
Yuan G, Yu Y, Li J, Jiang D, Gu J, Tang Y, Qiu H, Xiong W, Liu N (2021) Facile fabrication of a noval melamine derivative-doped UiO-66 composite for enhanced Co(II) removal from aqueous solution. J Mol Liq. https://doi.org/10.1016/j.molliq.2021.115484
Yuan G, Zhao C, Tu H, Li M, Liu J, Liao J, Yang Y, Yang J, Liu N (2018) Removal of Co(II) from aqueous solution with Zr-based magnetic metal-organic framework composite. Inorg Chim Acta 483:488–495. https://doi.org/10.1016/j.ica.2018.08.057
Li M, Yuan G, Zeng Y, Peng H, Yang Y, Liao J, Yang J, Liu N (2021) Efficient removal of Co(II) from aqueous solution by flexible metal-organic framework membranes. J Mol Liq 324:114718–114999. https://doi.org/10.1016/j.molliq.2020.114718
Yuan G, Tu H, Liu J, Zhao C, Liao J, Yang Y, Yang J, Liu N (2018) A novel ion-imprinted polymer induced by the glycylglycine modified metal-organic framework for the selective removal of Co(II) from aqueous solutions. Chem Eng J 333:280–288. https://doi.org/10.1016/j.cej.2017.09.123
Rada ZH, Abid HR, Sun H, Wang S (2015) Bifunctionalized metal organic frameworks, UiO-66-NO2-N (N = –NH2, –(OH)2, –(COOH)2), for enhanced adsorption and selectivity of CO2 and N2. J Chem Eng Data 60:2152–2161. https://doi.org/10.1021/acs.jced.5b00229
Zhao B, Yuan L, Wang Y, Duan T, Shi W (2021) Carboxylated UiO-66 tailored for U(VI) and Eu(III) trapping: from batch adsorption to dynamic column separation. ACS Appl Mater Interfaces 13:16300–16308. https://doi.org/10.1021/acsami.1c00364
Yuan G, Tian Y, Liu J, Tu H, Liao J, Yang J, Yang Y, Wang D, Liu N (2017) Schiff base anchored on metal-organic framework for Co (II) removal from aqueous solution. Chem Eng J 326:691–699. https://doi.org/10.1016/j.cej.2017.06.024
Silva A, Sousa KS, Germano A, Oliveira VV, Espínola J, Fonseca MG, Airoldi C, Arakaki T, Arakaki L (2009) A new organofunctionalized silica containing thioglycolic acid incorporated for divalent cations removal—a thermodyamic cation/basic center interaction. Colloids Surf A 332:144–149
Zhang X-F, Wang Z, Feng Y, Zhong Y, Liao J, Wang Y, Yao J (2018) Adsorptive desulfurization from the model fuels by functionalized UiO-66(Zr). Fuel 234:256–262. https://doi.org/10.1016/j.fuel.2018.07.035
Esrafili L, Firuzabadi FD, Morsali A, Hu ML (2021) Reuse of predesigned dual-functional metal organic frameworks (DF-MOFs) after heavy metal removal. J Hazard Mater 403:123696. https://doi.org/10.1016/j.jhazmat.2020.123696
Ali RM, Hamad HA, Hussein MM, Malash GF (2016) Potential of using green adsorbent of heavy metal removal from aqueous solutions: adsorption kinetics, isotherm, thermodynamic, mechanism and economic analysis. Ecol Eng 91:317–332. https://doi.org/10.1016/j.ecoleng.2016.03.015
Farnane M, Tounsadi H, Elmoubarki R, Mahjoubi FZ, Elhalil A, Saqrane S, Abdennouri M, Qourzal S, Barka N (2017) Alkaline treated carob shells as sustainable biosorbent for clean recovery of heavy metals: kinetics, equilibrium, ions interference and process optimisation. Ecol Eng 101:9–20. https://doi.org/10.1016/j.ecoleng.2017.01.012
Peres EC, Cunha JM, Dortzbacher GF, Pavan FA, Lima ÉC, Foletto EL, Dotto GL (2018) Treatment of leachates containing cobalt by adsorption on Spirulina sp. and activated charcoal. J Environ Chem Eng 6:677–685. https://doi.org/10.1016/j.jece.2017.12.060
Ferri M, Campisi S, Gervasini A (2019) Nickel and cobalt adsorption on hydroxyapatite: a study for the de-metalation of electronic industrial wastewaters. Adsorption. https://doi.org/10.1007/s10450-019-00066-w
Hashemian S, Saffari H, Ragabion S (2014) Adsorption of cobalt(II) from aqueous solutions by Fe3O4/bentonite nanocomposite. Water Air Soil Pollut. https://doi.org/10.1007/s11270-014-2212-6
Zhao P, Guo C, Zhang Y, Xiao Y, Wu X, Zhao Y (2016) Macroscopic and modeling evidence for competitive adsorption of Co(II) and Th(IV) on carbon nanofibers. J Mol Liq 224:1305–1310. https://doi.org/10.1016/j.molliq.2016.10.114
Jin Y, Wu Y, Cao J, Wu Y (2014) Adsorption behavior of Cr(VI), Ni(II), and Co(II) onto zeolite 13x. Desalin Water Treat 54:511–524. https://doi.org/10.1080/19443994.2014.883333
Al-Ghouti MA, Daana DA (2020) Guidelines for the use and interpretation of adsorption isotherm models: a review. J Hazard Mater 393:122383. https://doi.org/10.1016/j.jhazmat.2020.122383
Hanna SL, Rademacher DX, Hanson DJ, Islamoglu T, Olszewski AK, Nenoff TM, Farha OK (2020) Structural features of zirconium-based metal-organic frameworks affecting radiolytic stability. Ind Eng Chem Res 59:7520–7526. https://doi.org/10.1021/acs.iecr.9b06820
Acknowledgements
This work was supported by the National Natural Science Foundation of China (22106012 and 21876122), the Foundation of Key Laboratory of Radiation Physics and Technology of the Ministry of Education (2021SCURPT05), and the Natural Science Foundation of Chongqing (CSTB2022NSCQ-MSX1408 and CSTB2022BSXM-JSX0021).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yu, Y., Jiang, D., He, B. et al. Facile preparation of UiO-66 derivatives for the removal of Co(II) from aqueous solution: study on adsorption properties and irradiation stability. J Radioanal Nucl Chem 332, 4047–4056 (2023). https://doi.org/10.1007/s10967-023-09114-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-023-09114-w