Abstract
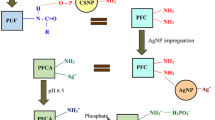
In this work, the Prussian blue nanorod was successfully synthesized and layer-by-layer deposited on polyurethane foams for removed strontium ions. The prepared composites were characterized by Fourier transform infrared (FT-IR), thermalgravimetric analysis (TG), atomic force microscope (AFM) and scanning electron microscopy (SEM). Prussian blue/Polyurethane foams (PB/PUF) exhibit huge surface area, pore volume and good resilience. PB/PUF displayed a maximum adsorption capacity of 45.15 mg/g for Sr2+ at room temperature. The pseudo-second-order equation was the best fit model for adsorption kinetic data compared with pseudo first-order and Elovich equation. The adsorption isotherm was investigated at 25, 30, and 35 °C, the data were in good agreement with the Freundlich isotherm. The thermodynamic parameters calculated by Van’t Hoff equation, the negative ΔG° indicated the adsorption process was spontaneous. Furthermore, the effect of co-existing ions on adsorption and the reusability of PB/PUF was also investigated. The PB/PUF presented high adsorption performance on Sr2+ and easy separation from wastewater. It is predicate that PB/PUF being an effective adsorbent for eliminating Strontium from radioactive wastewater.












Similar content being viewed by others
References
Shin J, Lee Y-G, Kwak J, Kim S, Lee S-H, Park Y, Lee S-D, Chon K (2021) Adsorption of radioactive strontium by pristine and magnetic biochars derived from spent coffee grounds. J Environ Chem Eng. https://doi.org/10.1016/j.jece.2021.105119
Goyal N, Gao P, Wang Z, Cheng S, Ok YS, Li G, Liu L (2020) Nanostructured chitosan/molecular sieve-4A an emergent material for the synergistic adsorption of radioactive major pollutants cesium and strontium. J Hazard Mater 392:122494. https://doi.org/10.1016/j.jhazmat.2020.122494
Yin J, Yang S, He W, Zhao T, Li C, Hua D (2021) Biogene-derived aerogels for simultaneously selective adsorption of uranium(VI) and strontium(II) by co-imprinting method. Sep Purif Technol. https://doi.org/10.1016/j.seppur.2021.118849
Bergbreiter DE, Chance BSJM (2007) “Click”-based covalent layer-by-layer assembly on polyethylene using water-soluble polymeric reagents. Macromolecules 40:5337–5343
Park B, Ghoreishian SM, Kim Y, Park BJ, Kang SM, Huh YS (2021) Dual-functional micro-adsorbents: Application for simultaneous adsorption of cesium and strontium. Chemosphere 263:128266. https://doi.org/10.1016/j.chemosphere.2020.128266
Jin T, Han Q, Wang Y, Jiao L (2018) 1D nanomaterials: design, synthesis, and applications in sodium-ion batteries. Small. https://doi.org/10.1002/smll.201703086
Cai D, Liu B, Wang D, Wang L, Liu Y, Qu B, Duan X, Li Q, Wang T (2016) Rational synthesis of metal–organic framework composites, hollow structures and their derived porous mixed metal oxide hollow structures. J Mater Chem A 4:183–192. https://doi.org/10.1039/c5ta07085f
Yang H-M, Park CW, Kim I, Yoon I-H (2020) Hollow flower-like titanium ferrocyanide structure for the highly efficient removal of radioactive cesium from water. Chem Eng J. https://doi.org/10.1016/j.cej.2019.123713
Ren W, Zhu Z, Qin M, Chen S, Yao X, Li Q, Xu X, Wei Q, Mai L, Zhao C (2019) Prussian white hierarchical nanotubes with surface-controlled charge storage for sodium-ion batteries. Adv Funct Mater. https://doi.org/10.1002/adfm.201806405
Ma M, Li W, Tong Z, Yang Y, Ma Y, Cui Z, Wang R, Lyu P, Huang W (2020) 1D flower-like Fe3O4@SiO2@MnO2 nanochains inducing RGO self-assembly into aerogels for high-efficient microwave absorption. Mater Des. https://doi.org/10.1016/j.matdes.2019.108462
Jain P, Pradeep T (2005) Potential of silver nanoparticle-coated polyurethane foam as an antibacterial water filter. Biotechnol Bioeng 90:59–63
Ariga K, Lvov Y, Decher G (2022) There is still plenty of room for layer-by-layer assembly for constructing nanoarchitectonics-based materials and devices. Phys Chem Chem Phys 24:4097–4115. https://doi.org/10.1039/D1CP04669A
Xu W, Ma X, Son JH, Jeong SY, Niu L, Xu C, Zhang S, Zhou Z, Gao J, Woo HY, Zhang J, Wang J, Zhang F (2021) Smart ternary strategy in promoting the performance of polymer solar cells based on bulk-heterojunction or Layer-By-Layer structure. Small. https://doi.org/10.1002/smll.202104215
He R, Dong C, Xu S, Liu C, Zhao S, He T (2022) Unprecedented Mg2+/Li+ separation using layer-by-layer based nanofiltration hollow fiber membranes. Desalination. https://doi.org/10.1016/j.desal.2021.115492
Pan H, Wang W, Pan Y, Song L, Hu Y, Liew KM (2015) Formation of layer-by-layer assembled titanate nanotubes filled coating on flexible polyurethane foam with improved flame retardant and smoke suppression properties. ACS Appl Mater Interfaces 7:101–111
Patra D, Vangal P, Cain AA, Cho C, Regev O, Grunlan JC (2014) Inorganic nanoparticle thin film that suppresses flammability of polyurethane with only a single electrostatically-assembled bilayer. ACS Appl Mater Interfaces 6:16903–16908
Tang Z, Wang Y, Podsiadlo P, Kotov NA (2006) Biomedical applications of layer-by-layer assembly: from biomimetics to tissue engineering. Adv Mater 18:3203–3224
Li Y, Yue Q, Gao B (2010) Adsorption kinetics and desorption of Cu(II) and Zn(II) from aqueous solution onto humic acid. J Hazard Mater 178:455–461. https://doi.org/10.1016/j.jhazmat.2010.01.103
Senthil Kumar P, Ramalingam S, Senthamarai C, Niranjanaa M, Vijayalakshmi P, Sivanesan S (2010) Adsorption of dye from aqueous solution by cashew nut shell: studies on equilibrium isotherm, kinetics and thermodynamics of interactions. Desalination 261:52–60. https://doi.org/10.1016/j.desal.2010.05.032
Wibowo E, Rokhmat M, Sutisna K, Abdullah M (2017) Reduction of seawater salinity by natural zeolite (Clinoptilolite): adsorption isotherms, thermodynamics and kinetics. Desalination 409:146–156. https://doi.org/10.1016/j.desal.2017.01.026
Zhang L, Wei J, Zhao X, Li F, Jiang F, Zhang M, Cheng X (2016) Removal of strontium(II) and cobalt(II) from acidic solution by manganese antimonate. Chem Eng J 302:733–743. https://doi.org/10.1016/j.cej.2016.05.040
Ahrouch M, Gatica JM, Draoui K, Bellido D, Vidal H (2019) Lead removal from aqueous solution by means of integral natural clays honeycomb monoliths. J Hazard Mater 365:519–530. https://doi.org/10.1016/j.jhazmat.2018.11.037
Cheng R, Kang M, Zhuang S, Shi L, Zheng X, Wang J (2019) Adsorption of Sr(II) from water by mercerized bacterial cellulose membrane modified with EDTA. J Hazard Mater 364:645–653. https://doi.org/10.1016/j.jhazmat.2018.10.083
Valsala TP, Joseph A, Sonar NL, Sonavane MS, Shah JG, Raj K, Venugopal V (2010) Separation of strontium from low level radioactive waste solutions using hydrous manganese dioxide composite materials. J Nucl Mater 404:138–143
Wu H, Lin S, Cheng X, Chen J, Ji Y, Xu D, Kang M (2020) Comparative study of strontium adsorption on muscovite, biotite and phlogopite. J Environ Radioact 225:106446. https://doi.org/10.1016/j.jenvrad.2020.106446
Mironyuk I, Tatarchuk T, Naushad M, Vasylyeva H, Mykytyn I (2019) Highly efficient adsorption of strontium ions by carbonated mesoporous TiO2. J Mol Liq 285:742–753. https://doi.org/10.1016/j.molliq.2019.04.111
Gunay A, Arslankaya E, Tosun I (2007) Lead removal from aqueous solution by natural and pretreated clinoptilolite: adsorption equilibrium and kinetics. J Hazard Mater 146:362–371. https://doi.org/10.1016/j.jhazmat.2006.12.034
Liu Y (2009) Is the free energy change of adsorption correctly calculated? J Chem Eng Data 54:1981–1985. https://doi.org/10.1021/je800661q
Zhang G, Xu X, Ji Q, Liu R, Liu H, Qu J, Li J (2017) Porous nanobimetallic Fe-Mn cubes with high valent Mn and highly efficient removal of arsenic(III). ACS Appl Mater Interfaces 9:14868–14877. https://doi.org/10.1021/acsami.7b02127
Datta SJ, Moon WK, Choi DY, Hwang IC, Yoon KB (2014) A novel vanadosilicate with hexadeca-coordinated Cs(+) ions as a highly effective Cs(+) remover. Angew Chem Int Ed Engl 53:7203–7208. https://doi.org/10.1002/anie.201402778
Acknowledgements
This work was supported by the “13th five plan” nuclear energy development and scientific research project (the fifth batch) project
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yao, C., Dai, Y. Layer-by-layer assembled Prussian blue compounds deposited on polyurethane foams for adsorption of strontium. J Radioanal Nucl Chem 332, 4113–4124 (2023). https://doi.org/10.1007/s10967-023-09103-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-023-09103-z