Abstract
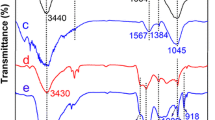
In this study, phosphorus-containing resin carbon (PRC) was prepared by freeze-drying and carbonization using sodium hexametaphosphate and a polymeric absorbent resin as raw materials. The adsorbents were characterized using infrared spectroscopy and scanning electron microscopy. The effects of pH value, adsorption time and temperature on uranium adsorption by PRC were investigated via static experiments. The adsorption process was analyzed via kinetic and isotherm models. The results showed that the equilibrium time of uranium adsorption by PRC was approximately 60 min, and the adsorption amount of uranium (VI) reached 909 mg g−1 at a pH of 6.0 and temperature of 313.15 K.







Similar content being viewed by others
References
Sarkar A (2019) Nuclear power and uranium mining: current global perspectives and emerging public health risks. J Public Health Policy 40:383–392. https://doi.org/10.1057/s41271-019-00177-2
Chen T, Yu KF, Dong CX, Yuan X, Gong X, Lian J, Cao X, Li MZ, Zhou L, Hu BW, He R, Zhu WK, Wang XK (2022) Advanced photocatalysts for uranium extraction: elaborate design and future perspectives. Coord Chem Rev. https://doi.org/10.1016/j.ccr.2022.214615
Guo H, Mei P, Xiao JT, Huang XS, Ishag A, Sun YB (2021) Carbon materials for extraction of uranium from seawater. Chemosphere. https://doi.org/10.1016/j.chemosphere.2021.130411
Hu B, Wang H, Liu R, Qiu M (2021) Highly efficient U(VI) capture by amidoxime/carbon nitride composites: evidence of EXAFS and modeling. Chemosphere. https://doi.org/10.1016/j.chemosphere.2021.129743
Cheng Y, He P, Dong F, Nie X, Ding C, Wang S, Zhang Y, Liu H, Zhou S (2019) Polyamine and amidoxime groups modified bifunctional polyacrylonitrile-based ion exchange fibers for highly efficient extraction of U(VI) from real uranium mine water. Chem Eng J 367:198–207. https://doi.org/10.1016/j.cej.2019.02.149
Wen S, Sun Y, Liu R, Chen L, Wang J, Peng S, Ma C, Yuan Y, Gong W, Wang N (2021) Supramolecularly poly(amidoxime)-loaded macroporous resin for fast uranium recovery from seawater and uranium-containing wastewater. ACS Appl Mater Interfaces 13:3246–3258. https://doi.org/10.1021/acsami.0c21046
Sen N, Darekar M, Sirsat P, Singh KK, Mukhopadhyay S, Shirsath SR, Shenoy KT (2019) Recovery of uranium from lean streams by extraction and direct precipitation in microchannels. Sep and Purif Technol. https://doi.org/10.1016/j.seppur.2019.05.083
Amphlett JTM, Choi S, Parry SA, Moon EM, Sharrad CA, Ogden MD (2020) Insights on uranium uptake mechanisms by ion exchange resins with chelating functionalities: Chelation vs. anion exchange. Chem Eng J. https://doi.org/10.1016/j.cej.2019.123712
Orrego P, Hernandez J, Reyes A (2019) Uranium and molybdenum recovery from copper leaching solutions using, ion exchange. Hydrometallurgy 184:116–122. https://doi.org/10.1016/j.hydromet.2018.12.021
Jiang H, Luo J, Liu Z, Liu S, Li F, Zuo L, Ma J, Luo M (2022) Porous nanofiber membrane from phase separation electronspun for selectively recovering uranium from seawater. J Radioanal Nucl Chem 331:2523–2532. https://doi.org/10.1007/s10967-022-08302-4
Shi S, Qian Y, Mei P, Yuan Y, Jia N, Dong M, Fan J, Guo Z, Wang N (2020) Robust flexible poly(amidoxime) porous network membranes for highly efficient uranium extraction from seawater. Nano Energy. https://doi.org/10.1016/j.nanoen.2020.104629
de Boulois HD, Joner EJ, Leyval C, Jakobsen I, Chen BD, Roos P, Thiry Y, Rufyikiri G, Delvaux B, Declerck S (2008) Impact of arbuscular mycorrhizal fungi on uranium accumulation by plants. J Environ Radioact 99:775–784. https://doi.org/10.1016/j.jenvrad.2007.10.009
Ren C-G, Kong C-C, Wang S-X, Xie Z-H (2019) Enhanced phytoremediation of uranium-contaminated soils by arbuscular mycorrhiza and rhizobium. Chemosphere 217:773–779. https://doi.org/10.1016/j.chemosphere.2018.11.085
Liu P, Yu Q, Xue Y, Chen J, Ma F (2020) Adsorption performance of U(VI) by amidoxime-based activated carbon. J Radioanal Nucl Chem 324:813–822. https://doi.org/10.1007/s10967-020-07111-x
Zhang F, Zhang H, Chen R, Liu Q, Liu J, Wang C, Sun Z, Wang J (2019) Mussel-inspired antifouling magnetic activated carbon for uranium recovery from simulated seawater. J Colloid Interface Sci 534:172–182. https://doi.org/10.1016/j.jcis.2018.09.023
Ahmad M, Ren J, Zhang Y, Kou H, M-u-d N, Zhang Q, Zhang B (2022) Simple and facile preparation of tunable chitosan tubular nanocomposite microspheres for fast uranium(VI) removal from seawater. Chem Eng J. https://doi.org/10.1016/j.cej.2021.130934
Li Y, Dai Y, Tao Q, Gao Z, Xu L (2022) Ultrahigh efficient and selective adsorption of U(VI) with amino acids-modified magnetic chitosan biosorbents: Performance and mechanism. Int J Biol Macromol 214:54–66. https://doi.org/10.1016/j.ijbiomac.2022.06.061
Lahiri S, Mishra A, Mandal D, Bhardwaj RL, Gogate PR (2021) Sonochemical recovery of uranium from nanosilica-based sorbent and its biohybrid. Ultrasonics Sonochem. https://doi.org/10.1016/j.ultsonch.2021.105667
Zhang F, Ma K-Q, Li Y, Ran Q, Yao C-Y, Yang C-T, Yu H-Z, Hu S, Peng S-M (2020) Selective separation of thorium from rare earths and uranium in acidic solutions by phosphorodiamidate-functionalized silica. Chem Eng J. https://doi.org/10.1016/j.cej.2019.123717
Yuan Y, Zhao S, Wen J, Wang D, Gu X, Xu L, Wang X, Wang N (2019) Rational design of porous nanofiber adsorbent by blow-spinning with ultrahigh uranium recovery capacity from seawater. Adv Funct Mater. https://doi.org/10.1002/adfm.201805380
Yuan Y, Yu Q, Wen J, Li C, Guo Z, Wang X, Wang N (2019) Ultrafast and highly selective uranium extraction from seawater by hydrogel-like spidroin-based protein fiber. Angewandte Chemie-International Edition 58:11785–11790. https://doi.org/10.1002/anie.201906191
Ma D, Wei J, Zhao Y, Chen Y, Tang S (2020) The removal of uranium using novel temperature sensitive urea-resin: and fast. Sci of the Total Environ. https://doi.org/10.1016/j.scitotenv.2020.139399
Tian W, Zhang H, Duan X, Sun H, Shao G, Wang S (2020) Porous carbons: structure-oriented design and versatile applications. Adv Funct Mater. https://doi.org/10.1002/adfm.201909265
Meng Y, Wang Y, Liu L, Ma F, Zhang C, Dong H (2022) MOF modified with copolymers containing carboxyl and amidoxime groups and high efficiency U (VI) extraction from seawater. Separ and Purif Technol. https://doi.org/10.1016/j.seppur.2022.120946
Zhang Y, Ye T, Wang Y, Zhou L, Liu Z (2021) Adsorption of uranium(VI) from aqueous solution by phosphorylated luffa rattan activated carbon. J Radioanal Nucl Chem 327:1267–1275. https://doi.org/10.1007/s10967-020-07592-w
Dhanya V, Arunraj B, Rajesh N (2022) Prospective application of phosphorylated carbon nanofibers with a high adsorption capacity for the sequestration of uranium from ground water. RSC Adv 12:13511–13522. https://doi.org/10.1039/d2ra02031a
Gan J, Zhang L, Wang Q, Xin Q, Hu E, Lei Z, Wang H (2023) Synergistic action of multiple functional groups enhanced uranium extraction from seawater of porous phosphorylated chitosan/coal-based activated carbon composite sponge. Desalination. https://doi.org/10.1016/j.desal.2022.116154
Kou Y, Zhang L, Liu B, Zhu L, Duan T (2020) Phosphonate modified MoS2 composite material for effective adsorption of uranium(VI) in aqueous solution. J Radioanal Nucl Chem 323:641–649. https://doi.org/10.1007/s10967-019-06970-3
Liu L, Lin X, Li M, Chu H, Wang H, Xie Y, Du Z, Liu M, Liang L, Gong H, Zhou J, Li Z, Luo X (2021) Microwave-assisted hydrothermal synthesis of carbon doped with phosphorus for uranium(VI) adsorption. J Radioanal Nucl Chem 327:73–89. https://doi.org/10.1007/s10967-020-07453-6
Wang Y, Yu C, Zeng D, Zhang Z, Cao X, Liu Y (2021) High-efficiency removal of U(VI) by mesoporous carbon functionalized with amino group. J Radioanal Nucl Chem 328:951–961. https://doi.org/10.1007/s10967-021-07747-3
Yunyun B, Jiang C, Liu Y, Wang C, Liu J (2021) Investigation of the adsorption properties of U(VI) by sulfonic acid-functionalized carbon materials. J Radioanal Nucl Chem 330:225–235. https://doi.org/10.1007/s10967-021-07952-0
Sen N, Singh KK, Mukhopadhyay S, Shenoy KT (2020) Continuous synthesis of tributyl phosphate in microreactor. Prog Nucl Energy. https://doi.org/10.1016/j.pnucene.2020.103402
Guo X, Feng Y, Ma L, Gao D, Jing J, Yu J, Sun H, Gong H, Zhang Y (2017) Phosphoryl functionalized mesoporous silica for uranium adsorption. Appl Surf Sci 402:53–60. https://doi.org/10.1016/j.apsusc.2017.01.050
Guo X, Feng Y, Ma L, Yu J, Jing J, Gao D, Khan AS, Gong H, Zhang Y (2018) Uranyl ion adsorption studies on synthesized phosphoryl functionalised MWCNTs: a mechanistic approach. J Radioanal Nucl Chem 316:397–409. https://doi.org/10.1007/s10967-018-5761-0
Song Q, Ma L, Liu J, Bai C, Geng J, Wang H, Li B, Wang L, Li S (2012) Preparation and adsorption performance of 5-azacytosine-functionalized hydrothermal carbon for selective solid-phase extraction of uranium. J Colloid Interface Sci 386:291–299. https://doi.org/10.1016/j.jcis.2012.07.070
Chen Z, He X, Li Q, Yang H, Liu Y, Wu L, Liu Z, Hu B, Wang X (2022) Low-temperature plasma induced phosphate groups onto coffee residue-derived porous carbon for efficient U(VI) extraction. J Environ Sci 122:1–13. https://doi.org/10.1016/j.jes.2021.10.003
Liu X, Li J, Wang X, Chen C, Wang X (2015) High performance of phosphate-functionalized graphene oxide for the selective adsorption of U(VI) from acidic solution. J Nucl Mater 466:56–64. https://doi.org/10.1016/j.jnucmat.2015.07.027
Liu Y, Zhao Z, Yuan D, Wang Y, Dai Y, Chew JW (2018) Fast and high amount of U(VI) uptake by functional magnetic carbon nanotubes with phosphate group. Ind Eng Chem Res 57:14551–14560. https://doi.org/10.1021/acs.iecr.8b03864
Ma D, Hu S, Li Y, Xu Z (2020) Adsorption of uranium on phosphoric acid-activated peanut shells. Sep Sci Technol 55:1623–1635. https://doi.org/10.1080/01496395.2019.1606016
Xin Q, Wang Q, Gan J, Lei Z, Hu E, Wang H, Wang H (2023) Enhanced performance in uranium extraction by the synergistic effect of functional groups on chitosan-based adsorbent. Carbohyd Polym. https://doi.org/10.1016/j.carbpol.2022.120270
Zhang Z-b, Zhou Z-w, Cao X-h, Liu Y-h, Xiong G-x, Liang P (2014) Removal of uranium(VI) from aqueous solutions by new phosphorus-containing carbon spheres synthesized via one-step hydrothermal carbonization of glucose in the presence of phosphoric acid. J Radioanal Nucl Chem 299:1479–1487. https://doi.org/10.1007/s10967-013-2830-2
Zou JZ, Niu Y, Tu WX, Zhang Q, Yao YC, Zeng SZ, Lan TB, Wu HL, Zeng XR (2019) Optimized synthesis of ultrahigh-surface-area and oxygen-doped carbon nanobelts for high cycle-stability lithium-sulfur batteries. J Electrochem Soc 166:A3464–A3473. https://doi.org/10.1149/2.1111914jes
Joshi RG, Gupta DK, Amesh P, Parida PK, Ravindran TR (2021) Microgel-hydrogel composite photonic crystals to monitor and extract uranyl ions in aqueous solutions. Microporous and Mesoporous Mater. https://doi.org/10.1016/j.micromeso.2021.111075
Niazi A (2006) Spectrophotometric simultaneous determination of uranium and thorium using partial least squares regression and orthogonal signal correction. J Braz Chem Soc 17:1020–1026. https://doi.org/10.1590/s0103-50532006000500029
Zhang C, Li X, Chen Z, Wen T, Huang S, Hayat T, Alsaedi A, Wang X (2018) Synthesis of ordered mesoporous carbonaceous materials and their highly efficient capture of uranium from solutions. Science China-Chem 61:281–293. https://doi.org/10.1007/s11426-017-9132-7
Husnain SM, Kim HJ, Um W, Chang Y-Y, Chang Y-S (2017) Superparamagnetic adsorbent based on phosphonate grafted mesoporous carbon for uranium removal. Ind Eng Chem Res 56:9821–9830. https://doi.org/10.1021/acs.iecr.7b01737
Dutta RK, Shaida MA, Singla K, Das D (2019) Highly efficient adsorptive removal of uranyl ions by a novel graphene oxide reduced by adenosine 5-monophosphate (RGO-AMP). J Mater Chem A 7:664–678. https://doi.org/10.1039/c8ta09746a
Liu R, Wang H, Yue C, Zhang X, Wang M, Liu L (2022) Synthesis of molybdenum disulfide/graphene oxide composites for effective removal of U (VI) from aqueous solutions. J Radioanal Nucl Chem 331:3713–3722. https://doi.org/10.1007/s10967-022-08425-8
El-Maghrabi HH, Younes AA, Salem AR, Rabie K, El-shereafy E-s (2019) Magnetically modified hydroxyapatite nanoparticles for the removal of uranium (VI): preparation, characterization and adsorption optimization. J Hazardous Mater. https://doi.org/10.1016/j.jhazmat.2019.05.096
Puziy AM, Poddubnaya OI, Gawdzik B, Tascon JMD (2020) Phosphorus-containing carbons: preparation, properties and utilization. Carbon 157:796–846. https://doi.org/10.1016/j.carbon.2019.10.018
Park J, Bae J, Jin K, Park J (2019) Carboxylate-functionalized organic nanocrystals for high-capacity uranium sorbents. J Hazard Mater 371:243–252. https://doi.org/10.1016/j.jhazmat.2019.03.007
Maity S, Bajpai S, Dhar BB (2021) Selective U(VI) removal using phosphorous-doped graphitic carbon. J Environ Chem Eng. https://doi.org/10.1016/j.jece.2020.104690
Yang P, Li S, Liu C, Shen C, Liu X (2021) Water-endurable intercalated graphene oxide adsorbent with highly efficient uranium capture from acidic wastewater. Sep and Purif Technol. https://doi.org/10.1016/j.seppur.2021.118364
Amesh P, Suneesh AS, Venkatesan KA, Chandra M, Ravindranath NA (2020) High capacity amidic succinic acid functionalized mesoporous silica for the adsorption of uranium. Colloids and Surf a-Physicochem and Eng Aspects. https://doi.org/10.1016/j.colsurfa.2020.125053
Guo D, Song X, Zhang L, Chen W, Chu D, Tan L (2020) Recovery of uranium (VI) from aqueous solutions by the polyethyleneimine-functionalized reduced graphene oxide/molybdenum disulfide composition aerogels. J Taiwan Inst Chem Eng 106:198–205. https://doi.org/10.1016/j.jtice.2019.09.029
Liu L, Yang W, Gu D, Zhao X, Pan Q (2019) In situ preparation of Chitosan/ZIF-8 composite beads for highly efficient removal of U(VI). Front Chem. https://doi.org/10.3389/fchem.2019.00607
Ju P, Liu Q, Zhang H, Chen R, Liu J, Yu J, Liu P, Zhang M, Wang J (2019) Hyperbranched topological swollen-layer constructs of multi-active sites polyacrylonitrile (PAN) adsorbent for uranium(VI) extraction from seawater. Chem Eng J 374:1204–1213. https://doi.org/10.1016/j.cej.2019.05.222
Ye T, Huang B, Wang Y, Zhou L, Liu Z (2020) Rapid removal of uranium(VI) using functionalized luffa rattan biochar from aqueous solution. Colloids and Surf a-Physicochem and Eng Aspects. https://doi.org/10.1016/j.colsurfa.2020.125480
Xiao F, Cheng Y, Zhou P, Chen S, Wang X, He P, Nie X, Dong F (2021) Fabrication of novel carboxyl and amidoxime groups modified luffa fiber for highly efficient removal of uranium(VI) from uranium mine water. J Environ Chem Eng. https://doi.org/10.1016/j.jece.2021.105681
Wang Y, Li Y, Zhang Y, Zhang Z, Li Y, Li W (2021) Nanocellulose aerogel for highly efficient adsorption of uranium (VI) from aqueous solution. Carbohyd Polym. https://doi.org/10.1016/j.carbpol.2021.118233
Sun Y, Wei Y, Pei J, Nan H, Wang Y, Cao X, Liu Y (2021) Study on adsorption of U(VI) from MOF-derived phosphorylated porous carbons. J Solid State Chem. https://doi.org/10.1016/j.jssc.2020.121792
Qin X, Yang W, Yang W, Ma Y, Li M, Chen C, Pan Q (2021) Covalent modification of ZIF-90 for uranium adsorption from seawater. Microporous and Mesoporous Mater. https://doi.org/10.1016/j.micromeso.2021.111231
Gong H, Lin X, Xie Y, Liu L, Zhou J, Liao H, Shang R, Luo X (2021) A novel self-crosslinked gel microspheres of Premna microphylla turcz leaves for the absorption of uranium. J Hazard Mater. https://doi.org/10.1016/j.jhazmat.2020.124151
Zhuang S, Wang J (2020) Poly amidoxime functionalized carbon nanotube as an efficient adsorbent for removal of uranium from aqueous solution. J Mol Liquids. https://doi.org/10.1016/j.molliq.2020.114288
Ahmed W, Mehmood S, Qaswar M, Ali S, Khan ZH, Ying H, Chen D-Y, Nunez-Delgado A (2021) Oxidized biochar obtained from rice straw as adsorbent to remove uranium (VI) from aqueous solutions. J Environ Chem Eng. https://doi.org/10.1016/j.jece.2021.105104
Li N, Gao P, Chen H, Li F, Wang Z (2022) Amidoxime modified Fe3O(4)@TiO2 particles for antibacterial and efficient uranium extraction from seawater. Chemosphere. https://doi.org/10.1016/j.chemosphere.2021.132137
Zhao C, Liu J, Deng Y, Tian Y, Zhang G, Liao J, Yang J, Yang Y, Liu N, Sun Q (2019) Uranium(VI) adsorption from aqueous solutions by microorganism-graphene oxide composites via an immobilization approach. J Clean Prod. https://doi.org/10.1016/j.jclepro.2019.117624
Acknowledgements
This work was supported by the National Innovation Training Program of China (202210555016).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yu, Y., Wan, Q. Adsorption of uranium (VI) in aqueous solutions by phosphorylated absorbent resin porous carbon. J Radioanal Nucl Chem 332, 4201–4211 (2023). https://doi.org/10.1007/s10967-023-09093-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-023-09093-y