Abstract
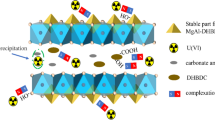
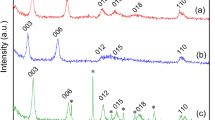
Two Mg–Al layered bimetallic (hydrogen) oxides with uranium selective deposited space were obtained by intercalating bimetallic (hydrogen) oxides with L-serine with active amino and carboxyl sites, which showed excellent uranyl selectivity from cation mixture solutions of Ni2+, Co2+, Sr2+, Zn2+, La3+, Gd3+, Ce3+, and Sn4+. It was firstly proved in L-serine intercalated Mg–Al layered bimetallic (hydrogen) oxides. The key parameter, maximum uranium uptake amount, reached 1028.37 mg/g for LS-LDH and 1018.50 mg/g for MgAl-LDO/C at pH 4.5. These merits coupling with very high uptake capacity, highly cost effectivity, facile preparation and acceptable recycle performance makes LS-LDH and MgAl-LDO/C promising in the treatment of uranium-containing solutions. The study enables the understanding of selectively treating uranium-containing wastewater with L-serine intercalated Mg–Al layered bimetallic (hydrogen) oxides.








Similar content being viewed by others
Data availability
Data available on request from the authors. The data that support the findings of this study are available from the corresponding author, upon reasonable request.
References
Wang S, Wang C, Yang XF, Yu JP, Tao WQ, Yang SL, Ren P, Yuan LY, Chai ZF, Shi WQ (2021) Selective separation of Am(III)/Eu(III) by the QL-DAPhen ligand under high acidity: extraction, spectroscopy, and theoretical calculations. Inorg Chem 60:19110–19119. https://doi.org/10.1021/acs.inorgchem.1c02916
Zhu L, Zhang L, Li J, Zhang D, Chen L, Sheng D, Yang S, Xiao C, Wang J, Chai Z, Albrecht-Schmitt TE, Wang S (2017) Selenium sequestration in a cationic layered rare earth hydroxide: a combined batch experiments and EXAFS investigation. Environ Sci Technol 51:8606–8615. https://doi.org/10.1021/acs.est.7b02006
Gao Z, Lai Y, Gong L, Zhang L, Xi S, Sun J, Zhang L, Luo F (2022) Robust Th-MOF-supported semirigid single-metal-site catalyst for an efficient acidic oxygen evolution reaction. ACS Catal 12:9101–9113. https://doi.org/10.1021/acscatal.2c02181
Liu X, Wang X, Jiang W, Zhang CR, Zhang L, Liang RP, Qiu JD (2022) Covalent organic framework modified carbon nanotubes for removal of uranium (VI) from mining wastewater. Chem Eng J. https://doi.org/10.1016/j.cej.2022.138062
Cheng XD, Chu J, Zhang LD, Suo ZR, Tang W (2022) Intracellular and extracellular untargeted metabolomics reveal the effect of acute uranium exposure in HK-2 cells. Toxicology. https://doi.org/10.1016/j.tox.2022.153196
Tillman FD, Beisner KR, Anderson JR, Unema JA (2021) An assessment of uranium in groundwater in the Grand Canyon region. Sci Rep. https://doi.org/10.1038/s41598-021-01621-8
Yuan Y, Yu Q, Wen J, Li C, Guo Z, Wang X, Wang N (2019) Ultrafast and highly selective uranium extraction from seawater by hydrogel-like spidroin-based protein fiber. Angew Chem-Int Ed 58:11785–11790. https://doi.org/10.1002/anie.201906191
Feng ML, Sarma D, Qi XH, Du KZ, Huang XY, Kanatzidis MG (2016) Efficient removal and recovery of uranium by a layered organic-inorganic hybrid thiostannate. J Am Chem Soc 138:12578–12585. https://doi.org/10.1021/jacs.6b07351
Xiong T, Li Q, Liao J, Zhang Y, Zhu W (2022) Highly enhanced adsorption performance to uranium (VI) by facile synthesized hydroxyapatite aerogel. J Hazard Mater 423:127184
Jana A, Unni A, Ravuru SS, Das A, Das D, Biswas S, Sheshadri H, De S (2022) In-situ polymerization into the basal spacing of LDH for selective and enhanced uranium adsorption: a case study with real life uranium alkaline leach liquor. Chem Eng J 428:131180
Lee HK, Chang S, Park W, Kim TJ, Park S, Jeon H (2022) Effective treatment of uranium-contaminated soil-washing effluent using precipitation/flocculation process for water reuse and solid waste disposal. J Water Process Eng. https://doi.org/10.1016/j.jwpe.2022.102890
Orabi AH, Mohamed BT, Ismaiel DA, Elyan SS (2021) Sequential separation and selective extraction of uranium and thorium from monazite sulfate leach liquor using dipropylamine extractant. Miner Eng. https://doi.org/10.1016/j.mineng.2021.107151
Dai Y, Cao B, Zhong S, Xie G, Wang Y, Liu Y, Zhang Z, Liu Y, Cao X (2019) Homogeneous liquid–liquid extraction of europium from aqueous solution with ionic liquids. J Radioanal Nucl Chem 319:1219–1225. https://doi.org/10.1007/s10967-019-06419-7
Gong X, Tang L, Zou J, Guo ZH, Li YL, Lei J, Liu HH, Liu M, Zhou L, Huang PL, Ruan HM, Lu YX, Zhu WK, He R (2022) Introduction of cation vacancies and iron doping into TiO2 enabling efficient uranium photoreduction. J Hazard Mater. https://doi.org/10.1016/j.jhazmat.2021.126935
Liang PL, Yuan LY, Deng H, Wang XC, Wang L, Li ZJ, Luo SZ, Shi WQ (2020) Photocatalytic reduction of uranium(VI) by magnetic ZnFe2O4 under visible light. Appl Catal B-Environ. https://doi.org/10.1016/j.apcatb.2020.118688
Darge AW, DeVol TA, Husson SM (2022) Phosphate-based reactive membranes for uranium isotopic screening. Ind Eng Chem Res 61:40. https://doi.org/10.1021/acs.iecr.2c02604
Reda AT, Zhang DX (2019) Sorption of metal ions from aqueous solution by sulfonated calix[4]arene intercalated with layered double hydroxide. J Environ Chem Eng 7:103021. https://doi.org/10.1016/j.jece.2019.103021
Wang H, Yao HQ, Chen LH, Yu ZH, Yang LX, Li C, Shi KR, Li CQ, Ma SL (2021) Highly efficient capture of uranium from seawater by layered double hydroxide composite with benzamidoxime. Sci Total Environ 759:143483. https://doi.org/10.1016/j.scitotenv.2020.143483
Li Y, Tang LP, Ma XX, Wang XR, Zhou W, Bai DS (2017) Synthesis and characterization of Zn–Ti layered double hydroxide intercalated with cinnamic acid for cosmetic application. J Phys Chem Solids 107:62–67. https://doi.org/10.1016/j.jpcs.2017.02.018
Mishra G, Dash B, Pandey S (2018) Layered double hydroxides: a brief review from fundamentals to application as evolving biomaterials. Appl Clay Sci 153:172–186. https://doi.org/10.1016/j.clay.2017.12.021
Zhang GG, Fang YG, Wang YD, Liu LJ, Mei DC, Ma FQ, Meng YJ, Dong HX, Zhang CH (2022) Synthesis of amino acid modified MIL-101 and efficient uranium adsorption from water. J Mol Liq. https://doi.org/10.1016/j.molliq.2021.118095
Li Y, Dai Y, Tao QQ, Gao Z, Xu L (2022) Ultrahigh efficient and selective adsorption of U(VI) with amino acids-modified magnetic chitosan biosorbents: performance and mechanism. Int J Biol Macromol 214:54–66. https://doi.org/10.1016/j.ijbiomac.2022.06.061
Li Y, Dai Y, Tao Q, Xu L (2022) Synthesis and characterization of amino acid-functionalized chitosan/poly(vinyl alcohol) for effective adsorption of uranium. J Radioanal Nucl Chem 331:4753–4765. https://doi.org/10.1007/s10967-022-08587-5
Tao Q, Xie J, Li Y, Dai Y, Liu Z (2022) Effects of dry processing on adsorption of uranium on Mg–Al layered double hydroxides and calcined layered double oxides. J Radioanal Nucl Chem 331:4587–4600. https://doi.org/10.1007/s10967-022-08529-1
Saleh TA (2022) Chapter 4-Isotherm models of adsorption processes on adsorbents and nanoadsorbents. In: Saleh TA (ed) Interface science and technology, vol 34. Elsevier, Netherlands, pp 99–126. https://doi.org/10.1016/B978-0-12-849876-7.00009-9
Muhire C, Zhang D, Xu X (2022) Adsorption of uranium (VI) ions by LDH intercalated with l-methionine in acidic water: Kinetics, thermodynamics and mechanisms. Results Eng 16:100686. https://doi.org/10.1016/j.rineng.2022.100686
Tu J, Peng X, Wang S, Tian C, Deng H, Dang Z, Lu G, Shi Z, Lin Z (2019) Effective capture of aqueous uranium from saline lake with magnesium-based binary and ternary layered double hydroxides. Sci Total Environ 677:556–563. https://doi.org/10.1016/j.scitotenv.2019.04.429
Ma S, Huang L, Ma L, Shim Y, Islam SM, Wang P, Zhao L-D, Wang S, Sun G, Yang X, Kanatzidis MG (2015) Efficient uranium capture by polysulfide/layered double hydroxide composites. J Am Chem Soc 137:3670–3677. https://doi.org/10.1021/jacs.5b00762
Yin L, Hu Y, Ma R, Wen T, Wang X, Hu B, Yu Z, Hayat T, Alsaedi A, Wang X (2019) Smart construction of mesoporous carbon templated hierarchical Mg–Al and Ni–Al layered double hydroxides for remarkably enhanced U(VI) management. Chem Eng J 359:1550–1562. https://doi.org/10.1016/j.cej.2018.11.017
Chen M, Li S, Li L, Jiang L, Ahmed Z, Dang Z, Wu P (2021) Memory effect induced the enhancement of uranium (VI) immobilization on low-cost MgAl-double oxide: mechanism insight and resources recovery. J Hazard Mater 401:123447. https://doi.org/10.1016/j.jhazmat.2020.123447
Asiabi H, Yamini Y, Shamsayei M (2018) Highly efficient capture and recovery of uranium by reusable layered double hydroxide intercalated with 2-mercaptoethanesulfonate. Chem Eng J 337:609–615. https://doi.org/10.1016/j.cej.2017.12.143
Acknowledgements
This study is financially supported by the National Natural Science Foundation of China (22066001) and the Natural Science Foundation of Jiangxi Province of China (20212ACB213001, 20224BAB203030).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
It should be understood that none of the authors have any financial or scientific conflicts of interest with regard to the research described in this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yusheng, W., Ying, D., Wenmei, H. et al. Uranium-specific adsorbent L-serine intercalated Mg–Al layered bimetallic (hydrogen) oxides for selectively treating uranium-containing wastewater. J Radioanal Nucl Chem 332, 3741–3752 (2023). https://doi.org/10.1007/s10967-023-09060-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-023-09060-7